October 2018
DR RONÉL HENDRIKS, chief scientific officer, ABM Africa
‛Soil health’ is a very widely used term in agriculture these days. Both producers and service providers have become increasingly soil health conscious. Soil health is not only an important component of your soil’s current productivity, but is also critical in the conservation of our food producing land (Di Cello et al., 1997).
Behind the term soil health there is a very complex interaction between living and non-living elements in the soil environment. When these elements are in balance, there are synergistic interactions and relationships that promote better soil and plant health.
Imbalances in the components involved in soil health result in a disease promoting environment where plants are stressed, disease levels are high and soil productivity is low (Garbeva et al., 2004).
When we look at the microbial component of soil health, we are dealing with microbial communities or microbiomes. Overly simplified, we can refer to them as ‘good’ communities (promoting soil and plant health) and ‘bad’ communities (suppressing beneficial microbes and causing disease).
Interestingly, the bad communities (disease complexes) have a very specific function in the soil environment, i.e. to recycle plants with weak genetics as food for the soil microbiome. It is important to distinguish between the two microbiomes found in soil since each of them has a unique contribution to soil and plant health (Table 1).

Bulk soil microbiome
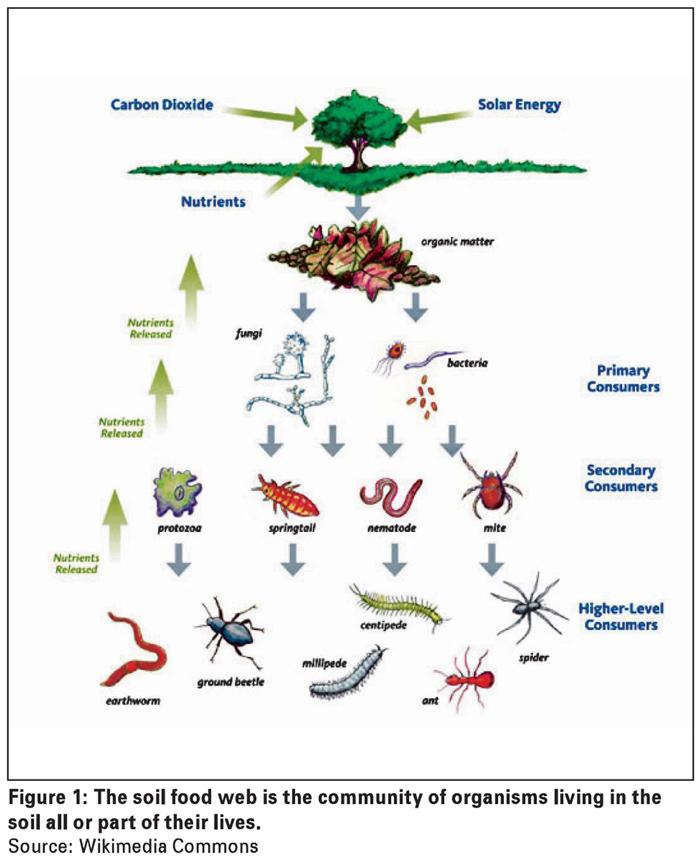
The bulk soil microbiome consists of communities of mostly free-living microbes, i.e. microbes not directly associated with plants. These microbes are all part of the soil food web. The soil food web, as presented in Figure 1, is a complex interaction of soil organisms (microbes, nematodes, insects, etc.) that each has a specific role in making nutrients available to the soil inhabitants and as a result has an impact on soil health.
Good soil health equals biodiversity. The extent of biodiversity has shown to be critical to the maintenance of soil health and quality. A large variety of microbial species results in a balanced and stable soil food web that is disease suppressive and plant growth promoting.
The opposite is an unbalanced food web where plant pathogens and parasitic nematode numbers are high due to the lack of natural enemies (bacterial and fungal feeders as well as predatory nematodes) (Garbeva et al., 2004; Wang et al., 2017a).
Factors to consider when the goal is to improve the microbial diversity in the soil are:
- Different soil environments select for different species and strains of a specific microbe. For example, mycorrhizae that live in the desert, will not grow in soil with higher clay content.
- Different soil management practices have different effects on the specific groups of the soil food web.
- Secretions from different plants stimulate growth of different microbial groups.
- Different organic soil amendments stimulate growth of different microbial groups.
 The rhizosphere microbiome
The rhizosphere microbiome
Plant roots release a wide range of compounds into the surrounding soil, including ethylene, sugars, amino acids, organic acids, vitamins, polysaccharides and enzymes. These root exudates create unique environments for the microbes living in close association with the roots, i.e. the rhizosphere.
A plant’s rhizosphere is a microbial hot spot and is considered one of the most complex ecosystems on earth (Jones and Hinsinger, 2008; Hinsinger et al., 2009; Raaijmakers et al., 2009). The rhizosphere composition as well as microbiome of different crops, e.g. maize and soybean, differ significantly (Wang et al., 2017b). Despite the diversity of microbes that is associated with the rhizosphere of different plants, one can distinguish between two main groups based on their relationship with the plant:
- Microbes that benefit from living (photosynthesising) plants.
- Microbes that benefit from dead (composting) plant material.
The first group of microbes obtains a major part of their carbon (sugar) from living, photosynthesising plants and as a result uses various mechanisms to keep plants healthy, stress-free and in an optimal state of photosynthesis. These microbes play an essential role in the viability, stress tolerance and productivity of a crop. Prominent members of the beneficial group are Bacillus, Trichoderma, Pseudomonas, Rhizobium, Azospirilium, Streptomyces, etc. (Mendes et al., 2013)
The second group of microbes that colonises the rhizosphere is microbes that obtain their carbon from dead plant material (cellulose). These microbial disease complexes target weak plants in order to recycle them as carbon for the soil food web. The ‘famous five’ that are part of this group are Fusarium, Phythium, Phytopthera, Rhizoctonia and plant parasitic nematodes (Whipps, 2001).
Healthy soil provides a good balance between beneficial microbes and pathogens. Unfortunately, most agricultural soils have high numbers of the pathogens and as a result plants are under constant attack when environmental or nutritional stresses surface.
In recent years, crop-specific, rhizosphere inoculums have provided a valuable tool in having a degree of control over who gets to the rhizosphere first. These inoculums consist of beneficial rhizosphere microbes that have the ability to establish in the rhizosphere (specifically the endo-rhizosphere) and form a synergistic relationship with each other as well as with the plant. When the bulk soil food web becomes healthier and more balanced, the effect of these inoculums becomes less prominent (Garbeva et al., 2004).
To summarise, the short-term strategy for improving plant health is to focus on the rhizosphere with crop-specific rhizosphere inoculums that can counter the imbalances between beneficial microbes and pathogen complexes. Soil health of the bulk soil on a specific farm and environment is a more complex long-term strategy that requires an individual plan based on the various factors involved.
References
Di Cello, F, Bevivino, A, Chiarini, L, Fani, R, Paffetti, D and Tabacchioni, S. 1997. Biodiversity of a Burkholderia cepacia population isolated from maize rhizosphere at different plant growth stages. Appl Environ Microbiol 63:4 485 – 4 493.
Garbeva, P, Van Veen, JA, and Van Elsas, JD. 2004. Microbial diversity in soil: Selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu Rev Phytopathol 42:243 – 270.
Mendes, R, Garbeva, P and Raaijmakers, JM. 2013. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev 37 (5):634 – 663.
Wang, P, Marsh, EL, Ainsworth, EA, Leakey, ADB, Sheflin, AM and Schachtman, DP. 2017b. Shifts in microbial communities in soil, rhizosphere and roots of two major crop systems under elevated CO2 and O3. Sci Rep 7:15019.
Wang, R, Zhang, H, Sun, L, Qi, G, Chen, S and Zhao, X. 2017a. Microbial community composition is related to soil biological and chemical properties and bacterial wilt outbreak. Sci Rep 7:343.
Whipps, JM. 2001. Microbial interactions and biocontrol in the rhizosphere. J Exp Bot 52 (1): 487 – 511.
Zhao, J, Liu, J, Liang, H, Huang, J, Chen, Z, Nie, Y, Wang, C and Wang, Y. 2018. Manipulation of the rhizosphere microbial community through application of a new bio-organic fertilizer improves watermelon quality and health. PLoS ONE 13(2): e0192967.

Publication: October 2018
Section: On farm level

















