April 2015
BRADLEY FLETT and EDSON NCUBE, ARC-Grain Crops Institute
Diplodia ear rot caused by Stenocarpella maydis, Fusarium ear rot caused by Fusarium verticillioides and Gibberella ear rot caused by the Fusarium graminearum species complex are the major ear rot diseases occurring in South Africa.
These diseases have been identified as recurring problems throughout maize producing areas. The fungi causing these diseases also cause maize stalk rots which result in plant lodging. Maize ear rots result in yield losses and grain quality reduction through discoloured kernels, observed during the grain grading process and production of mycotoxins.
Stenocarpella maydis, F. verticillioides and the Fusarium graminearum species complex produce mycotoxins that are toxic to humans and livestock. It is important to remember that environmental conditions favourable for ear rots do not correspond to conditions causing stalk rots, even though the same fungi are involved.
Each ear rot disease must be seen as an individual disease as climatic and/or environmental conditions for the development of each disease varies. These diseases will be discussed separately.
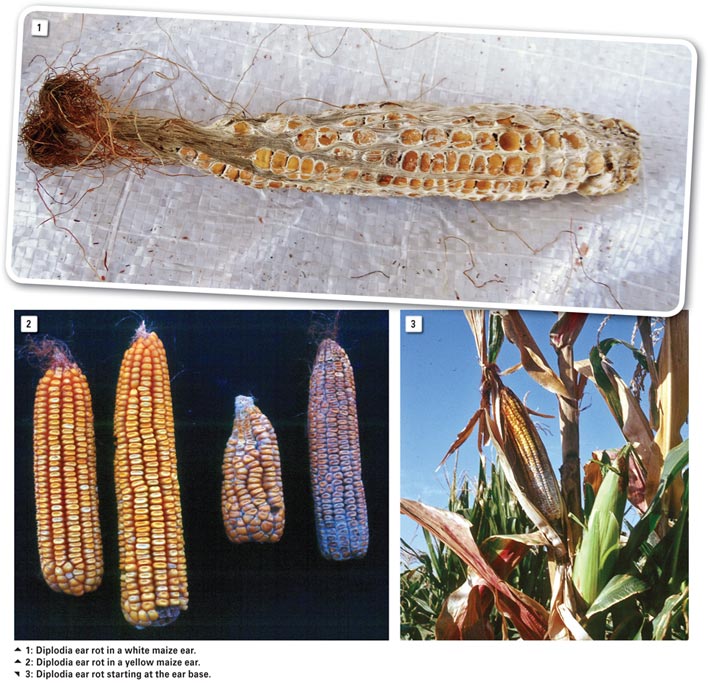
Diplodia ear rot
Symptoms in maize
Diplodia ear rot symptoms (Photo 1 and Photo 2) associated with infections during early ear development are the yellowing and drying of husk leaves while stalks and leaves remain green. Infection generally begins at the ear base and ramifies upwards (Photo 3). The entire ear becomes overgrown with a white mycelial growth.
A cross section of an infected ear shows black spore-producing bodies at the kernel bases. Late season infections may occur when kernel moisture is low and symptoms are less obvious. Embryos only become infected and slightly discoloured, but no ramification of the rest of the kernel occurs. Such symptomless infections are locally referred to as “skelm Diplodia”.
Life cycle and epidemiology
Stenocarpella maydis spores are transmitted by air, seed and soil. Airborne spores result in heavy infections of up to 10 m from the inoculum source and the number of successful infections is reduced with distance. Single spores travelling long distances may lead to trace infections which may then develop into an epidemic focal point. Infected maize seed is an important inoculum source which may result in seedling and crown rot diseases. However, the majority of S. maydis infected kernels do not germinate.
Spores land behind leaf or ear sheaths where they germinate and infect stalks or ears. Spore germination is inhibited by exposure to sunlight and desiccation. Free water is necessary for germination. Germination may take up to seven days and germinated spores may enter tissue and lie dormant until conditions are favourable for fungal growth through the tissues.
Mycelial colonisation is accompanied by cell wall degradation ahead of the growing pathogen. This is due to enzymes that are secreted. Fungal ramification of maize ears begins at the shank. Cob tissue colonisation begins at the attachment with embryonic tissues and proceeds into the endosperm. Similarly stalk ramification may also occur. These infected tissues develop fruiting bodies (pycnidia), which produce spores during the subsequent season.
Economic importance
Yield losses caused by diplodia ear rot have not yet been quantified because the harvest method, the make of the harvester, speed, and settings among other factors, all affect the percentage of rotten kernels that remain in the grain bin or trailer. Rotten kernels that are light are blown out during the harvesting process, the percentage depending on abovementioned factors.
This implies that where diplodia ear rot infections are serious, damage is twofold. Firstly, if a low percentage of rotten kernels are discarded during the harvesting process, serious grain quality reductions occur. Secondly, where a high percentage of rotten kernels are discarded in the harvest process, it will improve grain quality, but manifest itself as yield loss.
A number of mycotoxins have been isolated and identified from S. maydis infected grain. Diplodiatoxin, dipmatol and three chaetoglobosins have been found in S. maydis infected material. Recently diplonine, a neurotoxin produced by Stenocarpella maydis, was isolated and identified by researchers at the ARC-Grain Crops Institute (ARC-GCI) in collaboration with toxicologists at the ARC-Onderstepoort Veterinary Institute.
Planned research includes the development of techniques to identify and quantify these toxins in maize samples to determine the levels of each mycotoxin in various tissues produced by different isolates.
Not all S. maydis isolates are toxic. For example, two isolates from the same field may be fed to ducklings with one being toxic and the other not. This makes decisions difficult on whether or not to use Diplodia-infected grain for feed.
Milling infected grain is thought to reduce the heat-sensitive toxin and ensure that total Diplodia-infected grain content of feed rations is lower than 10%. Techniques enabling researchers to quantify the presence of these toxins will assist in determining which grain/isolates are toxic and which are not.
Animals, particularly cattle, being fed Diplodia-infected rations must be monitored. As soon as symptoms of reluctance to move, standing with wide-based stance, poor coordination, walking stifflegged with a high stepping gait, falling, paralysis, constipation, salivation and tremors are observed, animals must be removed immediately and fed on healthy rations. Recovery rates are high, but animals will die if kept on the infected ration.
Control measures
Stubble reduction
Control measures include reducing surface stubble by means of grazing, baling or ploughing-in of surface maize stubble on fields where high Diplodia levels have been identified. Since the S. maydis survives on maize stubble and survives poorly in soil, any management practice that reduces levels of infected surface stubble will reduce inoculum concentrations in the field.
The removal of stubble for a single season and then resorting back to stubble retention practices only reduces diplodia ear rot for that specific season. Where stubble is present the following season, the risk of Diplodia ear rot will increase to its original level, should weather conditions be favourable for it.
Crop rotation
Crop rotations reduce Diplodia ear rots by reducing inoculum levels in two ways. Firstly, a non-host for the fungus will not allow the fungus to persist for the season where maize is not grown. Secondly, a greater period (a season or two) between maize cropping allows for a natural breakdown of maize stubble, which again reduces the survival of the fungus. Leguminous crops such as soybeans, drybeans, groundnuts and cowpeas are very good rotational crops. Other rotational crops that reduce Diplodia ear rots are wheat and oats. Sunflowers do not significantly reduce Diplodia ear rots under experimental conditions, but the reason thereof has not yet been determined.
Early harvesting
Early harvesting will reduce Diplodia ear rots as it reduces time available for the fungus to grow on the ear. The fungus (Stenocarpella maydis) can grow on maize ears in the field until an 11% grain moisture content is reached.
Late or winter rains keep ears wet and increase the chance for fungal growth. In certain cases it would pay to harvest early at higher moisture levels and artificially dry grain. This possibly explains why Diplodia ear rot is not a major problem in the USA where maize is harvested early and dried artificially.
Hybrid resistance
Selection of hybrids is very important in the control of Diplodia ear rots. However, it appears that there is widespread confusion regarding the resistance and use of resistance. None of the hybrids on the market are resistant to Diplodia ear rot at all, however, some get more ear rots than others under specific climatic conditions. This reaction is affected by different climatic conditions which is important to consider when selecting resistant hybrids.
Fusarium ear rots
Symptoms
Fusarium ear rot (Photo 4 and Photo 5), is caused primarily by the fungus Fusarium verticillioides, formerly known as F. moniliforme. F. verticillioides also causes stalk and root rot, as well as seedling blight of maize.
Two major symptom types of this ear rot can be noted in the field. The first are symptoms observed in association with maize stalk borer feeding channels (Photo 6 and Photo 7).
F. verticillioides, in particular, is generally associated with insect or bird damage on maize ears. The fungus appears as pink/white mycelial growth on damaged kernels. The second symptom type is evident as pink or streaked kernel discolouration not related to kernel damage.
Fusarium verticillioides may infect kernels without showing any visible symptoms. It has been known for clean (first grade) grain samples to have symptomless infections of up to 90%.
Disease cycle
Fusarium verticillioides overwinters in maize debris and survives in maize stalks as thickened hyphae in moist soils that have poor aeration and little or no competition with other fungi and bacteria. The soil-borne hyphae germinate and infect the germinating seed and roots and move up the plant through systemic growth. The fungus also produces airborne spores from sporulation on the previous crop residue.
The mode of kernel infection by F. verticillioides is both through systemic infections from contaminated seed and through the silk channel by airborne spores. Silk colonisation by F. verticillioides starts from the tip of the ear downward.
Infection is enhanced by late-season rainfall and the physiological state of the silks after pollination. Direct invasion of kernels can also occur through weak points such as stress cracks in the pericarp and through the pedicel.
Insect transmission is primarily due to the stalk borers, Chilo partellus and Busseola fusca. Stalk borers feed on infected tissue, move to new plants or plant parts and continue feeding, while leaving the fungal spores in their frass. The fungus is released back to the soil through infected stalks or infected seed. Fusarium verticillioides is favoured by dry, hot climatic conditions such as those prevailing primarily in the north-western parts of the South African maize producing area.
Economic importance
Fusarium ear rot can result in yield and grade reductions. Infections associated with ear damage are often localised on cobs. The symptomless nature of certain infections by these fungi results in infected grain passing unnoticed. A major economic implication of Fusarium ear rot is the ability of these fungi to form mycotoxins in infected maize. The most important being fumonisins which are toxic to chickens, pigs and horses.
Horses are extremely sensitive to fumonisins and a level above five parts per million (ppm) in their feed will result in a fatal disease called leucoencephalomalacia or hole in the head syndrome. Guidance levels for fumonisins in pig and chicken feeds are set at maximum allowable (safe) levels of 10 ppm and 50 ppm, respectively. Research has also implicated this mycotoxin as one of the causes of human oesophageal cancer, which is common in certain regions of Africa, Europe, China and the USA.
Control measures
Control
Maize hybrids differ in their susceptibility to Fusarium ear rot. For example, studies currently underway at the ARC-GCI show that genetically modified maize hybrids that contain the insecticidal proteins for the control of maize stalk borer (Bt maize) have significantly less Fusarium ear rot symptoms compared to their non-Bt isohybrids.
Agricultural practices such as planting hybrids that are adapted to local climatic conditions, use of hybrids with tight husks, control of ear feeding insects, avoiding excessive plant populations, maintaining adequate levels of nitrogen and other essential growth nutrients, crop rotation and sub-soiling in compacted soils to minimise plant stress, are some of the possible means that can be followed to reduce Fusarium ear rot.
Standard grain storage procedures that prevent the development of fumonisin mycotoxins in stored grain, such as drying maize kernels to moisture levels below 16% after harvest, may be recommended where high infection levels are expected. Stored grain should be aerated regularly to lower moisture content and temperature to desired levels.
Adjusting the combine harvester to avoid kernel damage during harvesting reduces mycotoxin contamination. However, due to the common occurrence of these fungi in nature, the use of sanitation practices have not been very successful in disease reduction.
Graminearum ear rot
Symptoms
 Graminearum ear rot (Photo 8), also known as Gibberella or red ear rot, is caused by the fungus Fusarium graminearum and other fungi belonging to the Fusarium graminearum species complex which also causes root rot, crown rot, stalk rot and seedling blight of maize.
Graminearum ear rot (Photo 8), also known as Gibberella or red ear rot, is caused by the fungus Fusarium graminearum and other fungi belonging to the Fusarium graminearum species complex which also causes root rot, crown rot, stalk rot and seedling blight of maize.
Recent research has shown that three of the recently identified 16 species within the Fusarium graminearum species complex occur on maize roots and include F. boothii, F. meridionale and F. graminearum s.s. and only F. boothii occurs on maize ears in South Africa.
Disease symptoms are dark red discolouration of the whole or part of the maize ear. Early infections result in complete ear rotting, with husks adhering tightly to the ear. Graminearum ear rot usually progresses from the tip of the ear downward.
Survival
This fungus survives primarily on the surface of maize stubble throughout winter. Survival on other organic matter that may be in or on the soil has recently been shown to result in alternate sources for the survival of this pathogen. This includes crops such as lucerne and grass cover crops. This explains the presence of Gibberella ear and stalk rot on lands that have been cropped to “non-hosts” for a number of years.
Survival structures may develop and mature on organic material and/or maize stalk tissue under warm, wet conditions. Ascospores are exuded from the perithecia and are taken up into air currents, from where these spores can then be deposited on and infect other maize plants.
This fungus also infects various other cereals such as wheat and barley, which may help the pathogen to overwinter, causing even larger disease outbreaks the following season. Fusarium graminearum species complex infects maize seed and infection levels of up to 66% have been reported. Seed to seed transmission, however, has not been clearly shown.
Transmission
Spores produced in spore bearing bodies (perithecia) on maize stubble are transmitted via air currents. These spores infect the maize silks and grow down into the point of the ear. The pathogen has also been reported to be transmitted by birds and insects.
Climatic conditions
Graminearum ear rot severity is favoured by cool, wet weather within three weeks of silking. This disease is common under irrigation conditions in South Africa. Regions affected by sporadic outbreaks of this disease are generally KwaZulu-Natal and Mpumalanga and irrigation fields in the Limpopo and North West Province.
Economic importance
Graminearum ear rot is not as economically important as Fusarium ear rot in South Africa, but appears to be on the increase and may well become the most significant ear rot in South African maize production.
Reports from certain areas where cool, wet, late season conditions are experienced, imply sporadic and localised outbreaks of this ear rot disease. In certain cases severe yield and quality reductions were observed.
The major concern is toxicity, associated with this disease. F. boothii the ear rot infecting species of the Fusarium graminearum species complex in South Africa, is known to produce a number of important mycotoxins, which cause major problems for pig farmers especially.
These mycotoxins are zearalenone and deoxynivalenol and whilst there are also levels of nivalenol found in maize ear rot samples to date, we are not sure what the source of this mycotoxin is and how it gets into the grain.
F. boothii is a known deoxynivalenol producer and not able to produce nivalenol; which bodes the question of where does the nivalenol come from? Researchers at the ARC-GCI are presently working on this anomaly.
Toxigenic symptoms in pigs range from hormone induced syndrome caused by zearalenone, which reduces the reproductive performance of the animals, to feed refusal due to high levels of deoxynivalenol and/or nivalenol. Cattle appear to be much more resistant than pigs to the hormonal effects of zearalenone, whereas chickens do not seem to be affected. The most conspicuous changes in pigs due to zearalenone are enlargement of the uterus and mammary glands, and atrophy of the ovaries.
Feed refusal is apparently as a result of the unpalatability of the feed and may be reflected in decreased weight gains and slower growth rates. Vomiting may occur in animals that consume small quantities of infected grain. Maize containing more than 5% of infected kernels should not be included in rations for pigs, although it may be diluted with sufficient quantities of first grade maize.
Control measures
Crop rotation
Rotation of maize with non-graminaceous crops generally decreases the incidence of Graminearum ear rot. Recent studies have shown that the pathogens have the ability to colonise and survive on alternate crop organic matter left in or on the soil. It still is important to avoid crop rotations between graminaceous crops.
Stubble removal
As the fungus overwinters on maize stubble retained on the soil surface, the removal of maize residues will reduce disease incidence in the following crop season. Increased breakdown of organic matter will also reduce survival of the causal organisms. It is important to note that where maize is produced in conservation agriculture (CA) cropping systems; the retention of stubble is required which can increase the risk of maize ear rots. However, adhering to the CA principle of crop rotation will decrease the risk and is thus recommended as a standard practice.
 Cultivar selection
Cultivar selection
Reports in the literature indicate that hybrids vary in susceptibility to the disease. Local hybrids are currently being screened for resistance to this disease at the ARC-GCI.
For further information, the authors can be contacted on 018 299 6100.
Publication: April 2015
Section: On farm level