January 2017
Gibberella on maize, sorghum and wheat
ANEEN SCHOEMAN and SONIA-MARI GREYLING-JOUBERT, ARC-Grain Crops Institute, Potchefstroom
The three most important crops grown in South Africa are maize, sorghum and wheat. These crops are essential food sources (such as carbohydrates and vitamins) for humans and animals. They are also important in industrial products and therefore the cultivation of these crops are of eco-nomic importance. It assists in job creation, with export of products and helps to generate international currency.
Unfortunately, these crops can be infected by fungal pathogens that can influence the yield and also lead to economic losses. The whole plant can be infected – which can lead to root-, crown-, stemand ear rot (maize), grain mould (sorghum) and head blight (wheat).
The fungal pathogens that most commonly occur on these crops and can cause severe damage, belong to the genus Fusarium. Recently in South Africa there has been an increase in the occurrence of the Fusarium graminearum species complex infection of these three crops. The diseases caused by the Fusarium graminearum species complex are also known as Gibberella rot.
Previously it was thought that the diseases were caused by F. graminearum s.s., but with recent molecular technology new species were found. Up to date there have been 16 different fungal species that occur in the Fusarium graminearum species complex.
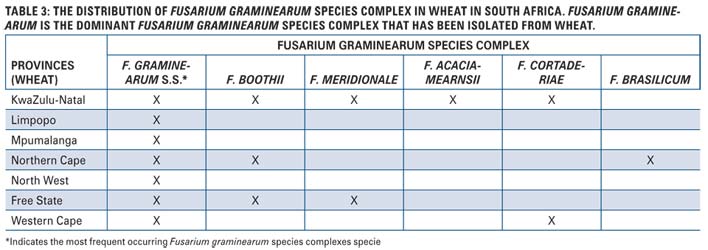
Another reason why the Fusarium graminearum species complex is important lies in the fact that these fungal pathogens can produce toxic products known as mycotoxins. The toxins that are produced are nivalenol (NIV) and deoxynivalenol (DON). Each fungal species in the Fusarium graminearum species complex can either produce DON or NIV and some species can even produce both toxins (Table 1).
It is possible that the health of people and animals can be negatively influenced when heavily mycotoxin-contaminated foodbased products of maize, sorghum or wheat are ingested over a long period of time. Up to date there has been no way to remove mycotoxin from contaminated food crops and the best way to reduce or limit mycotoxins in food is to control Fusarium graminearum species complex.
The fungal pathogens occurring in the Fusarium graminearum species complex can also differ in virulence (degree to which disease severity can occur), plant part specificity (some fungal pathogens can only infect roots/crowns/stems or above ground plant parts) and then some species display geographical specificity (occur only in specific areas in South Africa).
Research information gathered will help producers to be more aware of the Fusarium graminearum species complex occurring in their fields, as well as how to better control and prevent the infection.
Symptoms
Maize
Studies found that the fungal pathogen most likely infecting maize ears was F. boothii (Table 2), however, F. graminearum s.s. was also isolated a few times (Table 2). In the stems and roots these two species as well as F. meridionale were isolated (Table 2).
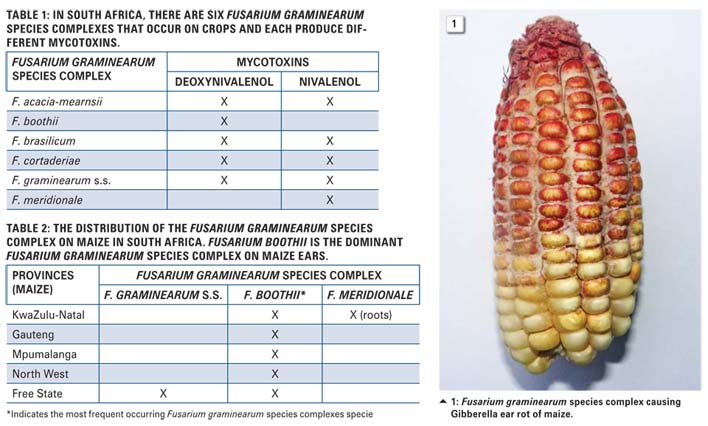
Visible fungal growth on a maize ear usually starts from the tip of the ear and spreads down to the base of the ear. Ears are more susceptible during grain filling stage and become less susceptible to infection as the ear matures.
Insects and birds that feed on the mature grain can expose the ear and make it more susceptible to infection of the fungal pathogen through the wounds. The visual symptoms showed that the grain turned a pinkish-red colour (Photo 1).
The discolouration affects the quality of the grain. Severely infected maize ears will lead to the grain not being suitable for food or feed. With stem rot there is usually the presence of leave damage. Infected stems and roots also show a characteristic pinkred discolouration when cut open. Severe stem- and root rots can lead to plants lodging in the field and leading to lower yields.
Wheat
In South Africa there are six Fusarium graminearum species complexes identified that infect wheat (Table 3). The fungal spores that occur on the plant debris in the field will infect the wheat rachis during flowering stage. A dark brown lesion becomes visible on a single spikelet and then the disease spreads to the spikelets via the rachis. The spikelets turn into a bleached colour and the tissue dies (Photo 2).
It is also possible that the pink-red fungal growth can be seen on the spikelets. The kernels will shrivel up and can also be covered with fungal growth (Photo 3). These kernels become light in weight and can be blown out during the harvest process from the harvester and leads to yield losses.
If these kernels are used in the new season, the seed will have poor germination which will result in seedling wilt. It is also possible to observe brown discolouration at the base of the stem and the rot will spread until the whole stem is rotten. This will lead to weak stems and the plant will lodge as in the case of maize.
Sorghum
The three Fusarium graminearum species complexes that infect sorghum in South Africa are F. meridionale, F. acacia-mearnsii and F. cortaderiae (Table 4).
The visual disease symptoms are quite difficult to identify and then to assign to a specific pathogen. The reason is that the visual symptoms are not as characteristic as on maize and wheat. The grain mould discolouration can be pink, grey, white or even black (Photo 4).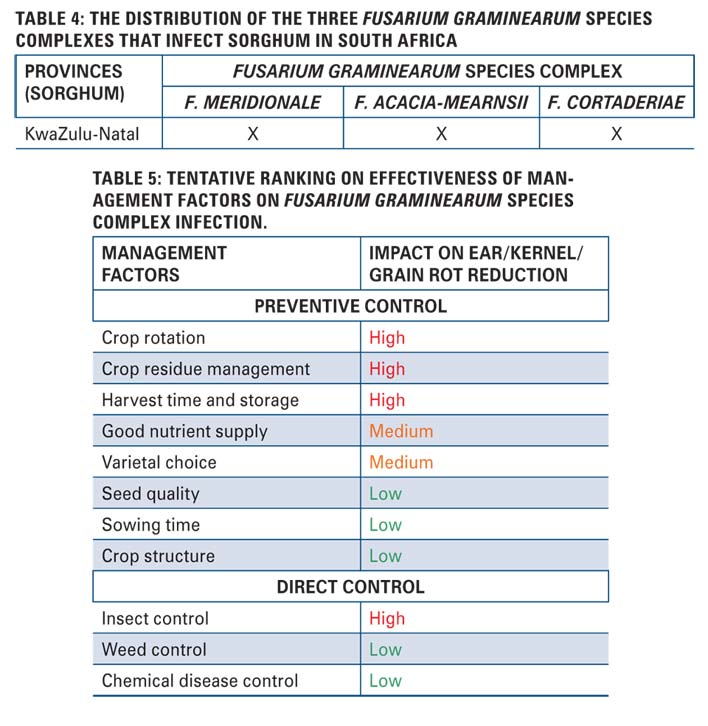
The stem rot symptoms are similar to stem rot of maize giving a characteristic pink or even purple discolouration of the internal stem tissue. Grain mould usually leads to loss of grain, severe grain discolouration, a decrease of grain size and weight and reduced market and nutritional value.
Control measures
Maize, wheat and sorghum each will have its own set of management strategies. Overall, achieving control of Fusarium graminearum species complex is very challenging and in most cases integrated management strategies are the best way to prevent infection of the crops. Each control strategy has a level of effectiveness towards the control of Fusarium graminearum species complex infection (Table 5).
Highly effective control strategies
Crop rotation
Fields that are grown in monoculture or rotated with similar crops result in crop residue from host plants, enabling the pathogens to overwinter on their crop of choice.
Crop residue management
Crop residue from the previous seasons will serve as the primary inoculum source for new infection in the growing season. In order to lessen disease, the residue can be physically removed, tilled into the field or be treated with microbial agents that aid in decomposition.
Harvest time and storage
Late harvest poses a high risk and it is therefore important to choose cultivars that are adapted to local climatic conditions. Moisture should be restricted to a minimum during storage, so grain can be dried to less than 15 %. Good storage practices such as appropriate temperature and moisture content, as well as insect control, aeration and clean bins should be maintained.
Insect control
Both the maize stalk borer (Busseola fusca) and spotted stalk borer (Chilo partellus) are linked to an increase in Fusarium spp. infection as the feeding wounds create openings for the pathogens.
Medium effective control strategies
Good nutrient supply
High levels of nitrogen and low levels of potassium can predispose the maize plant to Fusarium graminearum species complex infection.
Varietal choice
There seems to be two types of hybrids that are more susceptible to Fusarium and Gibberella ear rot, namely those cultivars with vertical ears that have poor ear cover and those with tight ear husks, respectively.
Low effective control strategies
Seed quality
Although seed quality has a minimal effect on Fusarium graminearum species complex infection, it should be of the best quality and free of disease.
Sowing time
Early planting usually escapes the worst infection.
Crop structure
High plant density favours disease development.
Weed control
Although weeds have a low effect on Fusarium graminearum species complex disease development, it may serve as an indirect control measure by increasing the humidity in the field.
Chemical disease control
Chemical control can be applied when Fusarium spp. systemically infects the maize plant early in the season. However, this does not work later in the season. Presently, there is no fungicide known to control Fusarium graminearum species complex.
Future research
Present research is performed in collaboration with researchers at Stellenbosch University in order to better understand the disease complex. The factors that are investigated include inoculum build-up (of the fungal isolates), pathogenicity of the Fusarium graminearum species complexes, epidemiology of species in the specific plant tissues, as well as development of management strategies.
The information will help to improve existing management strategies and will help to give more specific information to producers in order to prevent Fusarium graminearum species complex diseases to break out.
For more information, contact the plant pathologist at the ARC-GCI at 018 299 6100 or email Dr Aneen Schoeman at BelgroveA@arc.agric.za.
References
Boutigny, AL, Ward, TJ, Van Coller, G, Flett, BC, Lamprecht, SC, O’Donnell, K and Viljoen, A. 2011. Analysis of the Fusarium graminearum species complex from wheat, barley and maize in South Africa provides evidence of species-specific differences in host preference. Fungal Genetics and Biology, 48: 914 – 920. Czembor, E, Adamczyk, J, Posta, K, Oldenburg, E and Shürch, S. 2010. Prevention of ear rots due to Fusarium spp. on maize and mycotoxin accumulation, Maize Case Study – Guide number 3, pp 1 – 8. Lamprecht, S, Tewoldemedhin, YT, Botha, WJ and Calitz, F. 2011. Fusarium graminearum species complex associated with maize crowns and roots in the Kwa- Zulu-Natal province of South Africa. Plant Disease 95: 1 153 – 1 158. Mavhunga, M. 2013. Fusarium graminearum mycotoxins associated with grain mould of maize and sorghum in South Africa. MSc thesis, University of the Free State, Bloemfontein. 169 pp. Van Coller, GJ, Boutigny, AL, Rose, L, Ward, TJ, Lamprecht, SC and Viljoen, A. 2013. Fusarium spp. associated with head blight of wheat in South Africa. Acta Phytopathologica Sinica. 10th International Congress of Plant Pathology Abstracts 25 – 30 August, Beijing, China P07.010.
Publication: January 2017
Section: On farm level