September 2016
SCOTT SYDENHAM and HESTIA NIENABER, ARC-Small Grain Institute, Bethlehem
In a previous article the concept of making use of DNA markers to identify the presence of specific target-site mutations in herbicide resistant weeds has been explained extensively. Making use of these target-site DNA markers to identify resistance has proven to be much quicker, as opposed to herbicide damage characterisation in the glasshouse, which can take much longer.
This ultimately saves significantly on operational costs. Since molecular genotyping makes use of the plants’ DNA, it is also diagnostically more accurate and precise.
Additionally, these markers can identify cross-broad mutation patterns and distinguish between homozygous susceptible (wild type), heterozygous resistant (mutant) and homozygous resistant (mutant) plants through specific targeted codon target-site mutations.
The co-dominant ability of these markers allows for the heterozygous/ homozygous resistance status of samples to be determined. Heterozygous means that only one of the alleles is carrying the resistant mutation, whereas homozygous indicates that both alleles are carrying the resistance mutation.
If this year’s generation of ryegrass is heterozygous for a single target- site mutation in your field and those plants are left to make seed, a theoretical scenario could unfold, whereby 75% of the field will show high levels of resistance and only 25% will show some levels of control during the next season.
Uniquely, this molecular genotyping allows for the detection of resistance to different herbicide groups/classes within a single bulked sample or single plant, simultaneously. With the traditional her bicide damage characterisation in the glasshouse, this would be difficult if not impossible – especially on a single plant.
During the pilot phase of this weed resistance allele profiling (WRAP) service, the ARC-Small Grain Institute (ARC-SGI) asked producers to send in their bulked representative grass weed samples from problematic fields for testing at no cost. Importantly, fresh green leaf material is essential for extracting high quality DNA, emphasising the need for ARC-SGI to receive the material as quickly as possible.
In recent times, the result turnaround, from receiving samples to a fully detailed diagnostic report (with herbicide or weed management recommendations to the producer for specific fields) has been, on average, three to five working days. In some special cases, this result turnaround has been 48 to 72 hours after receiving samples. This service has now been running for three years.
In the 2015/2016 season, this service successfully screened 156 samples and provided 21 different client reports. Over the past few seasons, this service was optimised and fine-tuned even further.
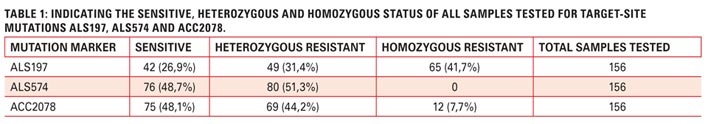
Highly reliable data can now be obtained of the most frequently occurring target-site mutations in South African ryegrass populations with the use of just three target-site mutation markers, i.e. ALS197, ALS574 and ACC2078, covering the most frequently occurring target-site mutations.
Results
ALS
Herbicides which inhibit Acetolactate Synthase (ALS) prevent the normal production of amino acids. Amino acids are the ‘building blocks’ for proteins needed for optimal plant growth and development. In the Herbicide Resistance Action Committee Chart, ALS or AHAS inhibitors are classified as Group B herbicides. This group includes the imidazolinones (IMI’s), sulfonylureas (SU’s) and trialzolopyrimidine (TP’s) herbicides.
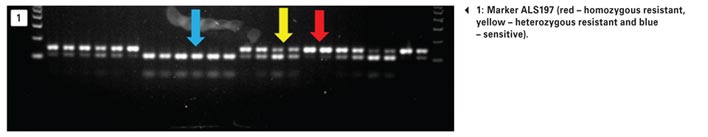
The ALS target site mutation 197 is the most common, present in 69% of the samples tested with marker ALS197 (Photo 1). This marker indicates the following resistance levels: Low to IMI’s, high to SU’s and low to TP’s.
The ALS 574 target site mutation was present in 51,3% of the samples tested. ALS574 mutation confers high levels of resistance to IMI’s, high to SU’s and low to TP’s. The presence of this marker combination makes all ALS herbicides non-effective. Limited, to no weed control would be achieved if this marker combination was present in your weed population. The heterozygous and homozygous statuses of both 197 and 574 are indicated in Table 1.
ACCase
Herbicides which inhibit lipid synthesis are known as ACCase inhibitors. In the HRAC chart ACCase inhibitor herbicides are classified as Group A herbicides. This group includes Aryloxyphenoxypropionates (‘fops’), Cyclohexanediones (‘dims’) and Pinoxaden (‘den’).
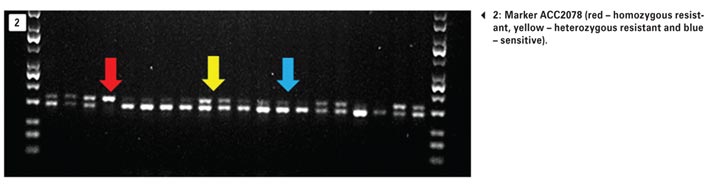
Currently, three markers are optimised to identify mutations of the ACCase herbicides. The 2078 ACCase target-site mutation occurred in 51,9% of the samples received (Photo 2). This mutation confers resistance to many ‘fops’ and all ‘dims’, including clethodim. A summary of the results of all tested samples to date can be seen in Table 1.
Conclusion
In the past season, 41% of the samples tested were positive for broad/cross-resistance to both ALS and ACCase herbicides. This meant no herbicides from the ALS or ACCase groups could be reliably recommended for the control of those producers’ resistant weed populations.
Alternative methods of weed management were recommended. Over the last year to 18 months, the resistant levels in samples tested have become progressively worse. The high levels of homozygous samples for ALS197 is of particular concern, implying that, if these populations are allowed to produce seed, practically 100% of that field could be resistant in the next season.
We believe the lack of detecting any homozygous sample for ALS574 is due a fitness factor/effect. From literature, there is evidence that an ALS574 mutation is rarely present in homozygous form as these plants do not survive.
The ‘growing’ presence of broad resistance to ACCase herbicides, is leaving the producers with very few post emergence chemical weed control options. The data presented underscore the harsh reality our producers (predominantly from the Western Cape, parts of the Eastern Cape and Northern Cape) are faced with.
How to get your ryegrass tested for target-site resistance?
Producers are welcome to send ryegrass seedlings to the ARC-SGI, Bethlehem. Please make sure that a representative sample is taken from plants over the entire field/multiple fields.
Seedlings must be kept moist and preferably couriered in zip-lock bags, as it will ensure that fresh seedlings arrive in Bethlehem. Carefully label the samples according to the field/farm they were collected from and indicate, if possible, the GPS-coordinates.
Courier samples to:
Hestia Nienaber/Scott Sydenham
ARC-Small Grain Institute
Blydskap Road, S191
Bethlehem
9700
Summary
From screening data so far obtained, it is evident that resistance to both ALS and/or ACCase inhibitor herbicides is a reality. However, by knowing which mutations are occurring in your field, informed choices can be made for the management of target-site resistant ryegrass.
 We would like to acknowledge the support received from the producers and chemical companies and encourage continuous future use of this WRAP service.
We would like to acknowledge the support received from the producers and chemical companies and encourage continuous future use of this WRAP service.
For further information regarding this topic or how to sample your weeds, contact Hestia Nienaber at 058 307 3420 or deweth@arc.agric.zaor sydenhams@arc.agric.za.
Publication: September 2016
Section: Focus on