Soil is the most fundamental resource for the farmer, without which food and natural fibre cannot be produced. This article forms part of a series to highlight this resource. The interaction of water in soil and plants is of exceptional importance for the crop farmer.
Soil is the medium that captures and retains water for plants and makes it available again to the plants over time. It also serves as a solvent for plant nutritional substances which then form the soil solution. In the next few articles in this series, water in the soil will be discussed. The energy state of water is the basis of water movement and retention in soil. In this article capillarity and the energy state of water in soil are discussed.
The water molecule
The structure of the water molecule is fundamental to its behaviour. The water molecule comprises one oxygen atom (O) and two hydrogen atoms (H) to form the chemical compound H2O. Of great importance is that the two hydrogen atoms are not diametrically opposite each other around the O atom, but both are found towards one side, forming an angle of 104,5° (Figure 1). The implication of this is that the water molecule is polarised. It is therefore slightly electro positive on the side of the two H atoms and slightly electro negative on the side of the O atom, while the water molecule itself is neutral.
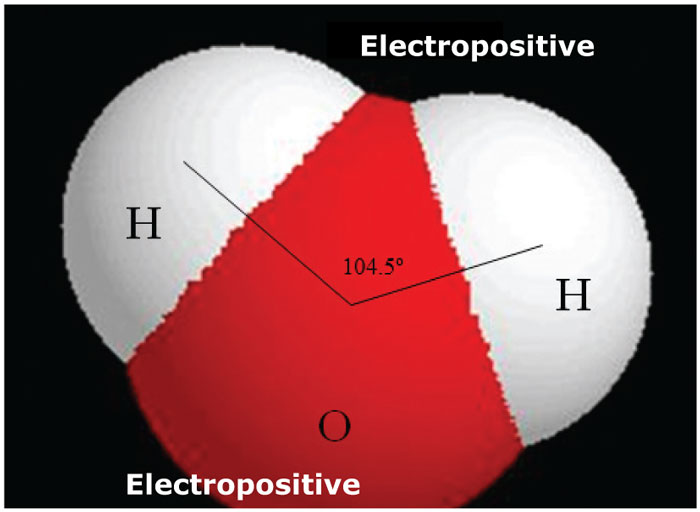
This polarity results in water molecules attracting each other electrostatically (cohesion). Amongst other things, cohesion causes surface tension, which is actually a thin dense elastic layer of water molecules (meniscus) on the surface of the water. Furthermore, the polarity property is also responsible for the concave meniscus of the water surface when, for example, it is in a glass tube (adhesion).
Surface tension and the meniscus are of major importance in soil, because they result in forces that retain water in the soil and allow it to move. The polarity property also causes ions such as H+, Na+, Mg2+and Ca2+ to be hydrated with water molecules. It also binds electrostatically with the negative clay surfaces to form a very thin layer of water molecules.
Capillary mechanism
Capillarity can be demonstrated when the one end of a narrow glass tube (or straw) is placed in a bowl of water and is held upright. Water moves upwards in the tube, contrary to the forces of gravity. The water molecules “creep” via adhesive forces (between the polar water molecule and the side of the glass tube) upwards and pull the next molecules with them using the cohesion forces (between water molecules). This movement continues until the attraction force of the earth (gravitation) on the water in the tube is equal to these forces and the water can no longer move further. The narrower the tube, the greater the curve of the meniscus, the stronger the force and the higher the water can move upwards. Exactly the same happens in the narrow tubes (pores) between soil particles where the water can then move upwards and sideways in the soil (Figure 2).
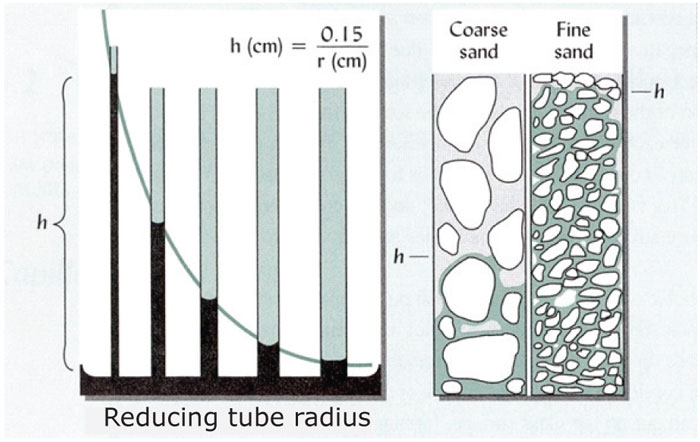
The pores in coarse sand are larger, with the result that the water can move more rapidly, but not very high (about 400 mm). In loam soils the pores are narrower and the water can move upwards as much as 800 mm, although more slowly. In clayey soils the water moves even more slowly and often not high, because clay usually has a strong structure without long continuous capillary tubes.
Energy state of water
Whenever an object moves from one place to another, work is performed and this requires energy. Water always moves from a higher energy level to a lower level. When the energy level in one place is the same as in another, movement will not take place. A number of factors result in various energy levels in soil. These energy levels provide the forces that allow water to move.
Gravitational potential
Gravitational potential refers to forces of gravity which draw the water downwards towards the centre of the earth. Water higher up in the soil is therefore constantly drawn downwards and thus deeper into the soil profile. Water that is higher in the profile therefore has a higher energy state than water deeper in the profile. Gravitational potential therefore causes the water, following rain or irrigation, to drain deeper into the soil and that excessive water drains deeply.
When free water occurs above the soil surface, it exercises a downwards and thus positive pressure on the water in the soil (also called overburden potential). It will therefore cause the energy level of water to increase and it will move more rapidly. The thicker the layer of water, the greater the water potential.
Matrix potential
Matrix potential is caused by adhesive and cohesive forces as well as menisci. Adhesive forces result in water molecules clinging to the soil particles in a thin dense layer and cohesive forces cause other water molecules to cling to this first layer, thus allowing “layers” of water molecules to form around the soil particles. Menisci form between these layers around the various soil particles and, together with the adhesive and cohesive forces, form the matrix potential (Figure 3a & b). The matrix potential is a negative value and is thus a suction force or suction potential.
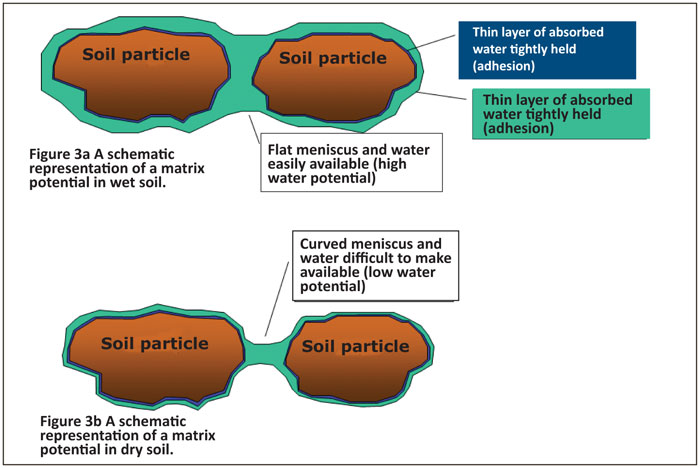
When the soil is wetted, the water moves downwards through the pores due to the gravity potential. Some of the water molecules cling to the soil particles due to adhesive forces which are stronger than the gravitational forces, thus remaining in the soil and not draining any deeper. This first layer of water molecules also holds a few layers of water molecules via cohesive forces, up to the point where the cohesive force is too weak (gravity too high) and the water then drains further downwards to a place where it is retained by the adhesive and cohesive forces or until it drains out of the profile.
Menisci form when two soil particles are found next to each other. Water clings to the soil particles and to each other, so that the water between the soil particles is continuous, with a meniscus that forms on the upper and lower sides (Figure 3). When the soil is wet, the meniscus is not greatly curved, but as the soil dries out, the curvature of the meniscus becomes greater and the forces become stronger. This then lowers the energy level of the water and therefore greater work must be done by gravity or a root to draw the water away from these forces.
In sandy soils theses forces are lower and the matrix potential lower (as the specific surface of sand is low – about 0,1 m2 g-1), and therefore sandy soils retain less water against gravity. The water which is retained is held “loosely” and is easily accessible for plants. In contrast, clayey soils have smaller particles and therefore a greater surface area (specific surface of as much as 800 m2 g-1). There are therefore higher adhesive forces, more layers for cohesive forces, more menisci and therefore a higher matrix potential. Clay can thus retain much more water in the profile against gravity, but also clings to much more water to such an extent that plants cannot absorb it.
The practical implication is therefore that (sandy) soil with a too low matrix potential loses a lot of its water to gravity, while clay soil with a high matrix potential clings to the water to such an extent that the plants cannot absorb it.
When one part of the soil profile is dry and another wet, the energy level between the two parts differs, with the wet portion having a higher energy level and the dry portion having a lower energy level. The water at the higher energy level will thus move towards the lower energy level. The water will continue to move towards the dry area, until the energy level of both parts is the same. The higher the difference in potential between the two parts and the closer they are to each other, the greater the potential gradient will be and the faster water will move.
Osmotic potential
Soil water contains a certain amount of dissolved organic and inorganic salts. These salts lower the energy level of the water as the water molecules are strongly attracted by hydration. The more salts in the soil water, the greater the osmotic potential (suction potential and thus a negative figure) and the greater the amount of work a plant root must do to draw water out of the soil against the osmotic forces. In saline soils with a large amount of salts, it could happen that a plant standing in wet soil dwilts.
Total soil water potential
The total soil water potential is calculated as the summation of the above mentioned water potentials. Some of them are positive while others are negative. When the total water potential between two adjoining bodies of soil differs, the water will move from the one with the higher potential to the one with the lower potential until the total water potential between the two is exactly the same. The rate of the movement will differ depending on the distance between these two points and the potential difference. Movement according to gravity potential is rapid (as much as 2 000 mm per day in sandy soil). Unsaturated flow (to be dealt with in later articles) due to matrix potential, is slow (about 1 mm to 2 mm per day).
Summary
In order to understand the movement of soil water, the forces applied to soil water is important. It amongst others, has an effect on the water retention of soil, the hydraulic conductivity, infiltration, provision of water to the plant roots, and the redistribution of water in soil. Each of these concepts will be dealt with in next articles.
For further information, please contact Martiens du Plessis at 072 285 5414 or martiens@nwk.co.za or Prof Cornie van Huyssteen at 051 401 9247 or vanhuysteencw@ufs.ac.za.
References
- Bennie, ATP. 1981. Soil Science 325. Unpublished class notes for GKD325. University of the Free State: Bloemfontein.
- Brady, NC. 1990. The nature and properties of soils. 10th ed. Macmillan Publishing Company: New York, USA.
- Van Huyssteen, CW. 2009. Soil Ecology. Unpublished class notes for GKD214. University of the Free State: Bloemfontein.

















