Soil is the most fundamental resource for the farmer, without which food and natural fibre cannot be produced. This article forms part of a series to highlight this resource. From a soil perspective, plant growth is determined by the availability of water and nutrients. The provision of water is dependent on the physical properties of the soil, while the chemistry of the soil determines the availability of plant nutrients. The soil’s chemistry is discussed in this and the following three articles.
Types of negative charges
The various clays and the charges that occur in them have already been discussed earlier. These clays or soil colloids have two types of negative charge. The first is the permanent charge that originates from isomorphic substitution. The second is the pH dependent charge, which forms when H+ and OH– groups in especially 1:1 clay, organic colloids, Fe and Mn hydroxides and certain amorphic compounds dissociate or protonise. The contribution of the constant and pH dependent charge of selected colloids is given in Table 1. The pH measures the concentration of H+ ions in the soil solution, on a scale from 1 to 14, with pH 7 as neutral. The lower the pH (<7), the higher the concentration of H+ ions in the soil solution. The pH dependent negative charge therefore increases as the pH increases and decreases as the pH decreases. The CEC of soils with a high fraction pH dependent charge will thus increase as the soil pH increases.
Table 1: The contribution of permanent and pH dependent charges to the cation exchange capacity (CEC) of selected soil colloids at pH 7
Type of soil colloid | Totale CEC cmolc kg-1 klei | Negative charges |
|
Permanent (%) | Variable(%) |
||
| Organic Matter | 240 | 25 | 75 |
| Montmorillonite (2:1) | 100 | 95 | 5 |
| Vermiculite | 85 | 100 | 0 |
| Illite | 30 | 60 | 40 |
| Kaolinite (1:1) | 10 | 5 | 95 |
| Gibbsite | 10 | 0 | 100 |
Positive charge
A limited positive charge of between 0 and 15 cmolc kg-1 could occur in highly weathered soils. This charge forms when cations with a low charge are replaced by cations with a higher charge, especially in highly weathered soils. A positive charge can also form by protonation at low pH:
AlOH + H+ → AlOH2+
Ion adsorption
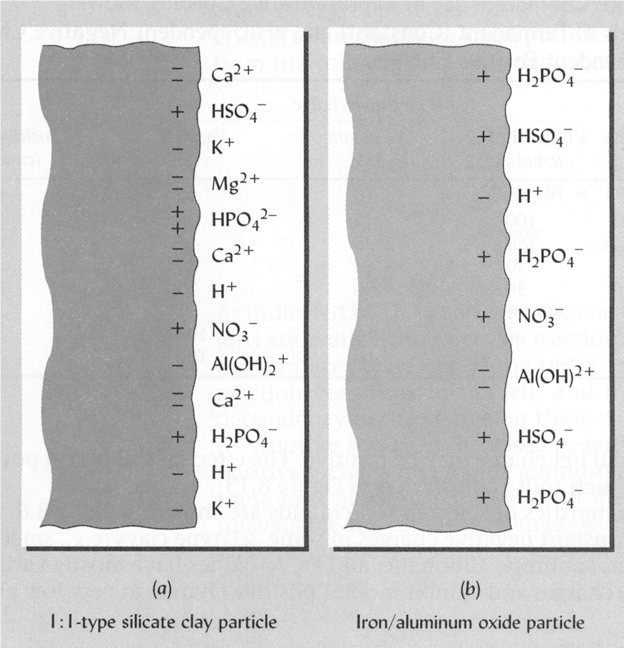
The negative and positive charges on the colloids must be balanced out with an opposite charge. A negative charge attracts positively charged cations such as Ca2+, Mg2+, K+ and H+, while a positive charge attracts negatively charged anions such as HPO42-, NO3- and SO42- (Figure 1)
Diffuse double layer
Ions are not bound in a single layer around the clay, but are spread in two layers around the clay. These two layers are jointly known as the diffuse double layer (Figure 2). Ions closest to the colloid are attracted strongest and form the innermost or Stern layer. The rest of the ions are more weakly bound and form the outer or diffuse layer, also known as the Gouy layer. Cation concentration is therefore the highest against the colloid and decreases further from the colloid. In contrast, the anion concentration is lowest close to the colloid and increases with distance. The boundary of the diffuse double layer is where the cation and anion concentration is equal. Cations and anions occur mixed in the diffuse double layer.
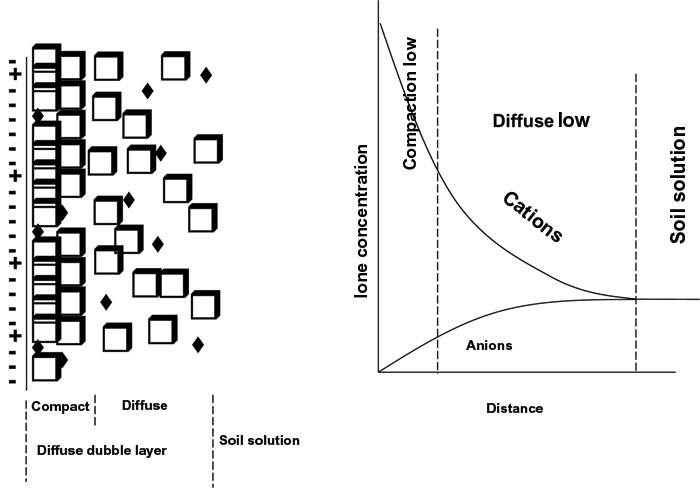
The thickness of the diffuse double layer is determined by the cation composition and concentration. Should the soil solution mainly comprise monovalent cations with a large ion radius (e.g. Na+), the diffuse double layer will be thicker than when the soil solution comprises cations with a higher charge and smaller ion radius (e.g. Ca2+). The thickness of the diffuse double layer is therefore also determined by the ion concentration. If the ion concentration be high, the double layer will be thin and if the ion concentration be low, the double layer will be thick.
The thickness of the diffuse double layer and the factors that influence it play a major role in the swelling and shrinkage properties of the soil, which takes place when the cations that are adsorbed in the colloids, adsorb water molecules, or when they dry out. Sodium adsorbs six times more water than calcium. The diffuse double layer will therefore be thick when sodium occurs predominantly in the soil. Similarly, a low salt concentration will lead to a thick diffuse double layer.
These two factors are of cardinal importance in saline and sodic soils, to be discussed in a future issue. When clay swells, the small particles are pushed away from each other. The natural structure of the soil is therefore broken apart, and the soil pore space decreases. The aeration and water movement in the soil is therefore reduced.
In conclusion, clay has a negative charge. This charge must be balanced by positively charged cations. These cations do not occur as a single layer, but are spread over two diffuse layers. The composition and concentration of cations in these layers determine the thickness of the diffuse double layer which, in turn, determines the structure stability and water movement in the soil. Cation exchange will be discussed in the next issue.
For further information, please contact Martiens du Plessis at 072 285 5414 or martiens@nwk.co.za or Cornie van Huyssteen at 051 401 9247 or vanhuysteencw@ufs.ac.za.
References
The following sources have been used extensively during the compilation of this article:
- Brady, NC. 1990. The nature and properties of soils. 10th ed. Macmillan publishing company: New York.
- Du T Burger, R. 1979. Soil Science 115. Unpublished class notes for GKD115. University of the Free State: Bloemfontein.
- Marshall, TJ and Holmes, JW. 1979. Soil Physics. Cambridge University Press: Cambridge.
- Van Huyssteen, CW. 2009. Soil Ecology. Unpublished class notes for GKD214. University of the Free State: Bloemfontein.

















