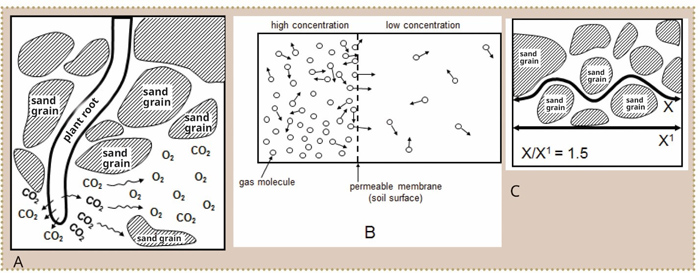
Soil is the most fundamental resource for the farmer, without which food and natural fibre cannot be produced. Because plant roots respire, the quantity of air in the soil is extremely important. Informed management of the factors responsible for the quantity and composition of the air in soil, is therefore essential for the prevention of plant damage due to water logging. In this issue we discuss the importance, composition and movement of air within the soil.
Plant roots and micro-organisms within the soil respire – they therefore need an electron acceptor during the oxidation of hydrocarbons to obtain energy. Plant roots and aerobic micro-organisms use oxygen, while anaerobic micro-organisms can use other elements such as nitrate (NO3-), manganese (MnO2) and iron (Fe2O3). Plant roots and aerobic micro-organisms thus use oxygen (O2) during respiration, while carbon dioxide (CO2) is produced. Should the oxygen concentration in the soil become too low, or the concentration of carbon dioxide become too high, the plant roots and micro-organisms cannot respire and they will die.
Composition of soil air
The CO2 concentration in the soil is normally eight times higher than in the atmosphere, while the O2 concentration is only slightly lower (Table 1).
Table 1: Average composition of soil and atmospheric air
| Element | Grondlug (%) | Atmosfeer |
|---|---|---|
| N2 | 79,2 | 79,0 |
| O2 | 20,6 | 20,9 |
| CO2 | 0,25 | 0,03 |
The pressure of water vapour in the soil air is close to 100%, compared with the considerably lower and varying values in the atmosphere. The nitrogen concentration is about the same in soil air as it is in the atmosphere. The higher CO2 and lower O2 concentration is due to the constant use of O2 and accompanying production of CO2. Soil pores make up about half of the volume of the soil, while the other half comprises the solid fraction (minerals and organic particles). The bulk density measures the ratio between the pores and solid particles (See Part 5 in this series). Soil air and water are found in the pores. They are mutually exclusive, therefore water will drive the air out of the soil.
Gas exchange
The excess CO2 in the soil needs to be exchanged with the O2 in the atmosphere. This process is known as gas exchange. In dry soil, almost all the pores are filled with air and gas exchange can take place easily. In moist soil, only the micro-pores are filled with water and gas exchange can readily take place through the macro pores. The wetter the soil becomes, the fewer the air-filled pores and the less they are linked together. This leads to a decrease in gas exchange, with a resultant increase in the CO2 and decrease in the O2 concentration.
These anaerobic conditions develop typically due to lengthy rains, over-irrigation, in low-lying areas in the landscape (wetlands), or where impenetrable layers occur in the soil. Under anaerobic conditions, anaerobic micro-organisms can use other compounds such as NO3–, MnO2, Fe2O3, SO42-, and even organic material as electron acceptors during respiration. Compounds such as methane (CH4), nitrogen oxide (NO), ethylene (CH2=CH2) and hydrogen sulphide (H2S) are then produced instead of CO2. These elements are harmful and even toxic for the majority of plants and are additionally responsible for plants dying under water saturated conditions.
Air movement in soil
Air can move through the soil via diffusion and mass flow. Diffusion, the more important of the two, is movement from a higher to a lower concentration, until equilibrium is reached (Figure 1). Equilibrium is, however, hardly ever reached in the soil, because O2 is constantly used and CO2 is constantly produced. Oxygen will therefore move by diffusion from a high O2 concentration near the surface of the soil (which is in contact with the atmosphere) to a low O2 concentration in the lower layers (near the plant roots). CO2 will diffuse the same way from the deeper horizons to the soil surface.
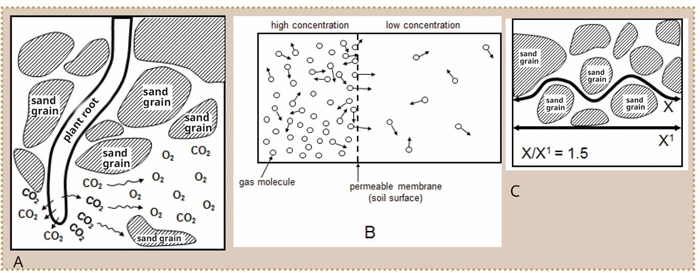
Diffusion takes place because of a difference in concentration (ΔC) over a certain distance (ΔZ). The greater the difference in concentration and the shorter the distance, the greater the diffusion. The difference in concentration divided by the distance ((ΔC/ΔZ) is known as the concentration gradient.
According to Fick’s Law, the flow of gas (q) is equal to the concentration gradient multiplied by a constant (D). This constant is known as the diffusion quotient of gas through soil.
q = D ΔC
ΔZ
The diffusion quotient of gas through soil is lower than in the atmosphere. Firstly because air-filled pores only make up 15% in moist soils, compared with the 100% in the atmosphere. Secondly, the gas molecules in the soil must follow a winding route (Figure 1). This winding route is about 1,5 times longer in soil than in the atmosphere and is known as tortuosity.
Mass flow is the movement of air from a higher to a lower pressure. Air pressure differences between the soil and the atmosphere seldom occur. It can occur when the temperature changes rapidly (e.g. during fires), the soil is wetted quickly, or when water is absorbed by plants or drains out of the soil. Air movement due to mass flow is therefore omissibly small.
Conclusion
The quantity and composition of soil air is therefore important because it determines the amount of oxygen available to plant roots for respiration. The composition of soil air can change drastically as a result of changes in soil water content, bulk density and the activities of micro-organisms. The precise management of these properties is therefore essential in preventing plant damage.
For further information, please contact Martiens du Plessis at 072 285 5414 or martiens@nwk.co.za or Prof Cornie van Huyssteen at 051 401 9247 or vanhuysteencw@ufs.ac.za.
REFERENCES
1. Bennie, ATP. 1981. Soil Science 354. Soil and water management. Unpublished class notes for GKD354. University of the Free State: Bloemfontein.