
Sandra Lamprecht,
ARC-Plant Health and Protection (ARC-PHP), Soilborne Plant Diseases Unit, Stellenbosch

Thabo Phasoana,
ARC-Plant Health and Protection (ARC-PHP), Soilborne Plant Diseases Unit, Stellenbosch

Anushka Gokul,
ARC-Plant Health and Protection (ARC-PHP), Soilborne Plant Diseases Unit, Stellenbosch

Chris Spies,
ARC-Plant Health and Protection (ARC-PHP), Soilborne Plant Diseases Unit, Stellenbosch
Sunflower is grown in many parts around the globe and is considered one of the most important oilseed crops in the world. It is also an important crop in South Africa and 678 000 tons was produced on 515 350 ha in 2019 locally.
The most important production areas are in the Free State, Limpopo and North West. Poor establishment is a major yield-limiting factor of sunflower production in South Africa (Figure 1). Although the contribution of other factors, such as seedling vigour, seedbed preparation and soil temperature, have been investigated, prior to the current study no information on the role of seedling diseases as a production constraint in sunflower production in South Africa was available.
Factors such as seedling vigour, plant depth, seedbed preparation and soil temperature have previously been identified as possibly contributing to poor establishment of sunflower locally. However, seedling diseases have not been considered to play a role in poor seedling establishment. This is most probably because information on seedling diseases of sunflower prior to the current study was almost non-existent in South Africa.
Research conducted on soilborne diseases of maize and soybean in South Africa, and on sunflower in other countries, showed that these field crops are susceptible to many soilborne pathogens that can cause damping-off and root rot, thus contributing to poor establishment. A study was therefore initiated to determine the role of seedling diseases in poor establishment of sunflower seedlings locally.
Areas surveyed and sampling
Field trials were established with untreated and treated (fludioxonil, mefenoxam and thiamethoxam) cultivar PAN 7120 CL seed during 2014/2015 and 2015/2016 at six localities in the Free State, Limpopo, Mpumalanga and North West. Sampling was conducted six weeks after planting and seedlings were also sampled from producers’ fields in these provinces. Both seedlings and soil were collected from each sampling site for isolations and glasshouse bioassays.

Disease symptoms
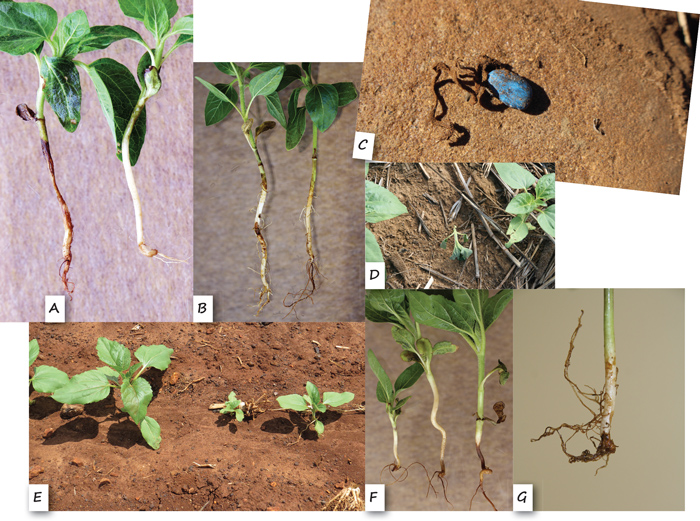
Lesions were often observed on hypocotyls and cotyledons of seedlings, and cotyledons were sometimes totally rotten (Figure 2A and 2B). Dead seedlings or seedlings dying, and pre-emergence damping-off were quite rare (Figure 2C and 2D). However, stunted seedlings (Figure 2E) were recorded at all localities, and these seedlings often displayed root rot symptoms and the tap roots were often stunted (Figure 2F and 2G).
Pathogens associated with diseased seedlings
Isolations were made from diseased seedlings that were collected from all the areas sampled. At least 31 fungal genera, which included more than 94 fungal species, were obtained from diseased cotyledons, hypocotyls and roots. A large number of isolates representing each species from the different genera were tested to determine their importance in causing disease on sunflower seedlings under glasshouse conditions. The effects of the standard seed treatment (fludioxonil, mefenoxam and thiamethoxam) against the pathogens that were identified, were also evaluated. The most important pathogens were species within Alternaria, Diaporthe, Fusarium, Macrophomina, Pythium and Rhizoctonia which caused pre- and post-emergence damping-off, hypocotyl/stem rot, a reduction in growth and root rot.
Alternaria
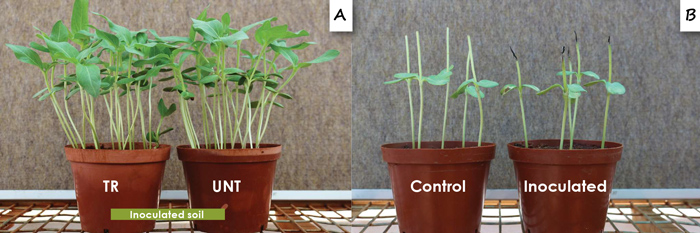
Alternaria species were isolated from seedlings from all localities. Alternaria alternata and A. tenuissima were obtained from seedlings. The predominant species isolated was A. alternata and very few A. tenuissima isolates were obtained. The Alternaria spp. were more frequently isolated from seedlings from untreated seed than treated seed and were significantly more frequently isolated from the cotyledons and hypocotyls than the roots. Glasshouse tests showed that A. alternata was the more aggressive of the two species, with certain isolates causing significant root rot (although at low severities). However, none of the two species significantly reduced survival of seedlings or caused a reduction in growth when planted in soil infested with the pathogen (Figure 3A).
Many of the isolates also caused lesions on stems (Figure 3B). Research conducted in other countries showed that seedborne A. alternata can reduce seed germination and cause seedling mortality. However, seed infected with A. alternata and tested by the ARC-PHP under glasshouse conditions did not reduce seedling survival. Alternaria alternata was also reported by other researchers to cause leaf blight of sunflower in South Africa.
Diaporthe
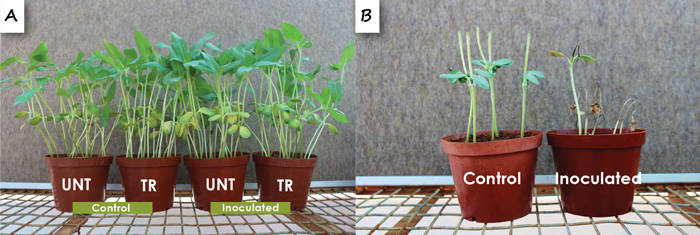
Diaporthe species were isolated from seedlings collected in all the provinces. They were more prominent on seedlings in North West compared to the other three provinces and were also significantly more frequently obtained from hypocotyls than cotyledons or roots of seedlings. Seed treatment did not affect the incidence of these fungi in seedlings. Four species were obtained and one of these is currently being described as a new species. Diaporthe gulyae was the most frequently isolated species and also the most virulent when tested for pathogenicity on seedlings under glasshouse conditions. The fungus did not affect seedling survival or growth and also did not cause root rot (Figure 4A). However, it caused quite severe die-back of stems of seedlings under glasshouse conditions (Figure 4B). Diaporthe species are important stem canker pathogens of sunflower in other countries. The isolation of these fungi from sunflower seedlings may have important implications not only for seedling health, but also for stem canker on older plants.
Fusarium
At least 38 Fusarium species or species complexes were isolated from sunflower seedlings. Fusarium species/species complexes were one of the predominant groups obtained from the seedlings and occurred on seedlings in all the provinces. The most frequently isolated species were species complexes of Fusarium incarnatum–equiseti (FIESC), F. oxysporum (FOSC) and F. solani (FSSC). FIESC was isolated more frequently from cotyledons than hypocotyls and roots, whereas FOSC and FSSC were isolated much more frequently from the roots than cotyledons or hypocotyls of seedlings and incidences were higher on seedlings from untreated than treated seed.
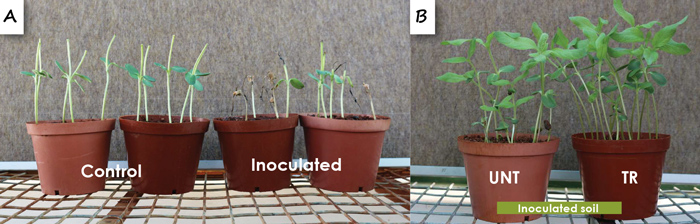
Many of the species caused a slight reduction in survival and low root rot severities. However, a species within the Fusarium solani species complex caused a significant reduction in survival and growth and severe root rot that killed the seedlings. Seed treatment was not effective to manage this pathogen (Figure 5A and 5B). Certain of the Fusarium species, such as F. andiyazi and F. verticillioides also caused a stunting of the taproots of seedlings (Figure 5C). Many Fusarium spp., including F. equiseti, F. oxysporum, F. solani and F. verticillioides, have been associated with diseases of sunflower seedlings in other countries and the importance of Fusarium spp. as pathogens of sunflower seems to increase worldwide.

Macrophomina
Macrophomina phaseolina was frequently isolated from seedlings – significantly more frequently from roots than cotyledons and hypocotyls. There was no difference in the incidence of the fungus on seedlings from treated and untreated seed planted in field trials. The fungus was frequently obtained from localities in the Free State, Limpopo and North West provinces. Glasshouse tests evaluating 50 isolates for their ability to cause disease on seedlings, showed that the fungus, except for one isolate, did not cause a reduction in survival and caused more severe aboveground symptoms than root rot.
The seed treatment significantly improved survival of seedlings in soil infested with the isolate that caused a reduction in survival and root rot of seedlings (Figure 6A and 6B). It is known that M. phaseolina can be both soil- and seedborne, is an important pathogen of sunflower and can cause charcoal rot and root rot. If seedborne, the fungus can reduce germination and vigour of sunflower seed and cause damping-off and seedling blight. The seed used in the ARC-PHP trials were tested and were not infested with the fungus.
Pythium
Pythium species were obtained from seedlings from all provinces and more frequently from seedling roots than cotyledons and hypocotyls. There was no significant difference in the incidences of Pythium spp. in seedlings from treated and untreated seed. Six Pythium species were obtained from the seedlings that were collected, with the most virulent species being P. irregulare and P. ultimum var. ultimum. Pythium irregulare significantly reduced survival and growth of seedlings (Figure 7A), and caused cotyledon and root rot (Figure 7B) and P. ultimum var. ultimum only caused root rot, but significantly less than P. irregulare.
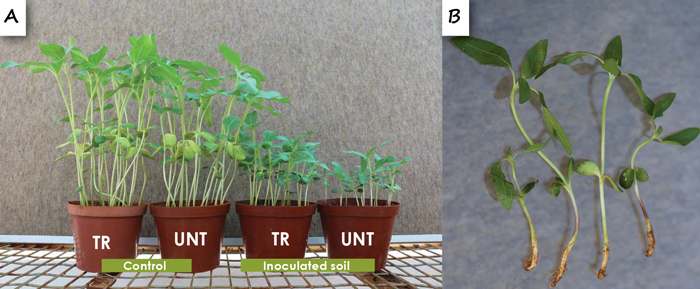
Although seed treatment significantly improved survival and growth and reduced cotyledon and root rot of seedlings in soil inoculated with P. irregulare, seedlings from treated seed were still stunted (Figure 7A). Information on Pythium spp. as pathogens of sunflower seedlings in other countries is very limited. Information thus far showed that it is possible that Pythium spp., especially P. irregulare, is one of the pathogens that may contribute to the stunted growth of sunflower seedlings that is quite common in many sunflower fields.
Rhizoctonia
Rhizoctonia anastomosis groups were obtained from all the localities sampled. There was no difference in the incidence of Rhizoctonia obtained from seedlings from treated and untreated seed and these fungi were isolated from cotyledons, hypocotyls and roots of seedlings. 14 Rhizoctonia anastomosis groups were obtained from sunflower seedlings and four of these were pathogenic on sunflower seedlings, namely AG 2-2LP, AG 4 HGI, AG 4 HGIII and an unidentified anastomosis group temporarily labelled as SB3.
These anastomosis groups caused a severe reduction in survival of seedlings and hypocotyl and root rot, with AG 4 HGI and SB3 being the most virulent (Figure 8A and 8B). The seed treatment was quite effective in controlling AG 2-2LP, AG 4 HGIII and SB3. However, although the seed treatment improved survival and reduced hypocotyl and root rot of seedlings in soil inoculated with AG 4 HGI, it was still not satisfactory (Figure 8A).
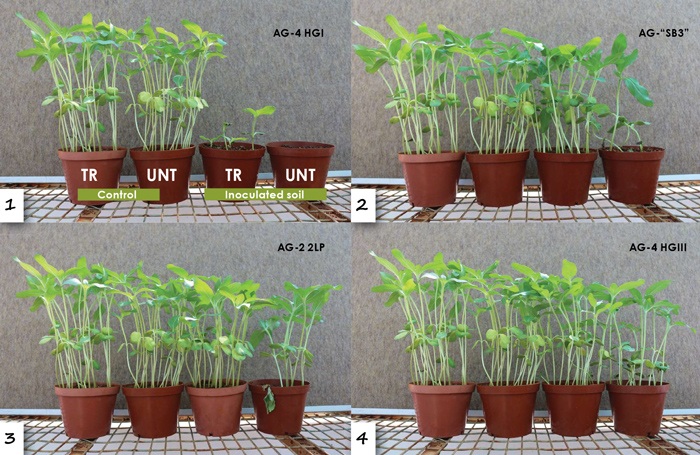

This anastomosis group was obtained from seedlings collected from North West, whereas SB3 was obtained from seedlings from all provinces. The information on Rhizoctonia anastomosis groups as pathogens of sunflower in other countries is very limited, but it appears that it may contribute significantly to poor establishment of sunflower seedlings in South Africa.
Conclusion
The results of the surveys and glasshouse studies showed that many fungal species including important pathogens are associated with sunflower seedlings and that certain fungi were more prevalent in certain localities than others. It clearly shows that seedling diseases are caused by a complex of pathogens. These can significantly reduce the survival and growth of seedlings as well as cause cotyledon, hypocotyl and root rot and can therefore contribute to poor establishment. Pathogenicity studies identified the most important pathogens.
Many of the pathogens identified have a broad host range and cannot be controlled with crop rotation. Since crop rotation is such an important part of conservation agriculture, crops that are susceptible to some of the same pathogens such as maize, sunflower and soybean are often rotated in the same field. Effective seed treatments can play a significant role as part of an integrated management strategy to protect sunflower against pathogens with a broad host range. The results obtained from seed treatment trials under glasshouse conditions showed that although the standard seed treatment (which targets a broad range of pathogens) is effective against many of the pathogens, it is unfortunately not effective against all of the virulent pathogens that were identified. Proper establishment of seedlings is very important to improve yield and an essential component of sustainable production. Research is therefore currently conducted to evaluate a range of seed treatments to identify the most effective treatment that will target most of the important pathogens responsible for poor establishment of sunflower seedlings in South Africa.
Acknowledgements
The authors wish to thank the Oil and Protein Seed Development Trust, the Oilseeds Advisory Committee and ARC-PHP for financial assistance; Dr Andre Nel from ARC-GCI and Ruaan Lochner, Abre Pretorius and Louis Schoonraad from Pannar for sunflower seed, planting and maintaining the trials and assisting with the sampling; producers for permission to sample their sunflower fields and technical personnel of the Soilborne Plant Diseases Unit of the ARC-PHP in Stellenbosch for excellent technical assistance.



















