
van Rensburg,
ARC-Grain Crops, Potchefstroom
Maize with a high yield potential are often more susceptible to certain diseases, resulting in the use of prophylactic fungicide spray programmes. Since 2007 a significant increase in the use of fungicides in maize production has become prominent to prevent and/or control foliar diseases. An increase in the market price of maize has also helped to make fungicide sprays economically viable.
Such spray programmes usually include a combination of strobilurin and triazole at the five-leaf to eight-leaf stage of plant growth, followed by a second spray of triazole 28 days to 30 days after the first spray. No fungicides are currently registered to control Fusarium ear rot diseases and their subsequent mycotoxins. The aim of this study is to determine whether prophylactic fungicide regimes for foliar diseases could reduce the risk of colonisation of grains by Fusarium verticillioides (Photo 1) and F. boothii (Photo 2) and their resultant mycotoxin production.
 F. verticillioides and F. boothii are two of the predominant maize fungal ear rots that can cause a reduction in grain quality as well as the production of mycotoxins that can be detrimental to humans and animals. F. verticillioides can produce fumonisins B1, B2, and B3 and F. boothii can produce the estrogenic metabolite zearalenone (ZEA) together with the nivalenol (NIV) and deoxynivalenol (DON) mycotoxins.
F. verticillioides and F. boothii are two of the predominant maize fungal ear rots that can cause a reduction in grain quality as well as the production of mycotoxins that can be detrimental to humans and animals. F. verticillioides can produce fumonisins B1, B2, and B3 and F. boothii can produce the estrogenic metabolite zearalenone (ZEA) together with the nivalenol (NIV) and deoxynivalenol (DON) mycotoxins.
Randomised split-split plot trials were conducted during the 2018/2019 planting season under dry land conditions with three replicates per treatment at Potchefstroom (warm, dry production area in the North West Province), Cedara (subtropical production area in KwaZulu-Natal) and Vaalharts (semi-desert area in the Northern Cape). The whole-plot factor represented days of spray applications and the second main effect, spray combinations. The sub-plot effect was cultivar. Two maize cultivars (BG3292 and BG3492B) were used and trials were maintained according to best practice appropriate to the respective production areas. Experimental plots were monitored for the five-leaf to twelve-leaf, pre-flowering, flowering and soft dough stages of plant growth.

Active ingredients from the triazole, strobilurin and benzimidazole fungicide classes, currently being used by producers as prophylactic fungicides for the prevention and control of foliar diseases, were tested in various combinations (Table 1). The controls included naturally infected plants with no fungicide treatment. Four rows of each cultivar in an experimental block were planted and the middle two rows were sprayed to prevent fungicide drift to other experimental blocks. The outer two rows represented the controls. During silking, maize ears of one middle row was inoculated with F. verticillioides and the other middle row with F. boothii. Plants were scouted throughout for leaf diseases and stalk borers. Plots were individually harvested and rated, threshed and milled. Samples were subjected to qPCR and HPLC analyses to determine the amount of fungal DNA present in grain samples and to quantify different mycotoxins, respectively. These trials were repeated in the 2019/2020 season and the results are currently being analysed. Therefore, the preliminary results of the 2018/2019 season are discussed in this article.
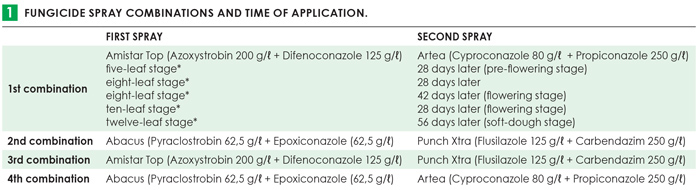
Results showed that the inoculation method was effective, as target DNA fungal biomass was significantly higher compared to the naturally infected plants. Infection of maize grain by F. verticillioides and F. boothii (target DNA) varied over the three localities and was the highest in Cedara and the lowest in Potchefstroom. In this study period, cultivar BG3292 was always more susceptible to fungal infection and mycotoxin production in maize grain, compared to its isoline BG3492B. The highest fumonisin levels were recorded at Potchefstroom in cultivar BG3292 and overall, DON and ZEA levels were quantified in low amounts. None of the mycotoxin levels exceeded South African regulatory limits. In some instances, only cultivar as main variable had an effect on mycotoxin production in maize grain.
Cedara
At Cedara, the mean F. verticillioides infection of maize grain was 1 444,96 pg µg-1 (considered as a high fungal infection). The Amistar Top/Artea (1335,2 pg µg-1), Abacus/Artea (1 140,5 pg µg-1) and Abacus/Punch Xtra (1 123,4 pg µg-1) spray combinations showed significantly (P=0,05) less fungal ear rot infection when compared to the Amistar Top/Punch Xtra spray combination (2 174,8 pg µg-1). Mean F. boothii infection of maize grain in Cedara was 256,31 pg µg-1 (considered as a low fungal infection). Anova results showed that only spray combination as main factor had a significant influence on F. boothii fungal infection of maize grain in Cedara (P=0,04). The Abacus/Punch Xtra (176,72 pg µg-1) and Abacus/Artea (189,01 pg µg-1) spray combinations had significantly less fungal infection of maize grain when compared to the Amistar Top/Artea (342,94 pg µg-1) and Amistar Top/Punch Xtra (318,02 pg µg-1) spray combinations. Anova results showed no significant effects regarding the spray combinations and dates of combinations on mycotoxin production in grain at Cedara.
Potchefstroom
In Potchefstroom mean F. verticillioides infection of maize grain was 39,43 pg µg-1. Maize ears harvested from the Amistar Top/Artea spray combination had significantly higher mean (P=0,05) F. verticillioides target DNA (54,21 pg µg-1) when compared to maize ears subjected to the Abacus/Artea (41,86 pg µg-1), Abacus/Punch Xtra (39,86 pg µg-1) and Amistar Top/Punch Xtra (32,47pg µg-1) spray combinations. Mean F. boothii infection of maize grain in Potchefstroom was 42,15 pg µg-1. The tested parameters had no effect on F. boothii infection or mycotoxin production of maize grain at Potchefstroom.
Vaalharts
Mean F. verticillioides infection of maize grain in Vaalharts was 175,49 pg µg-1. Cultivar BG3292 yielded significantly higher mean F. verticillioides infections and fumonisins (273,75 pg µg-1 and 3 074,9 µg/kg) compared to its isoline, cultivar BG3492B (78,06 pg µg-1 and 1002,0 µg/kg). Fungicide spray regimes had no effect on F. verticillioides ear rot infections or fumonisins. Mean target DNA levels of F. boothii in maize grain were 53,17 pg µg-1 in Vaalharts. F. boothii target DNA in maize grain was significantly higher (P<,0001) with 74,86 pg µg-1 in cultivar BG3292 compared to 32,02 pg µg-1 in cultivar BG3492B. The tested parameters had no effect on mycotoxin production of maize grain at Vaalharts.
This is the first time that it can be proved that certain fungicide spray combinations can reduce fungal infection (in Cedara and Potchefstroom), which is an added advantage for producers. The non-significance of application dates is also a positive finding, because producers do not need to add extra sprays to the existing prophylactic fungicide regimes (to include a later spray), which means that extra costs are avoided. 
It must be noted that in an environment conducive for fungal infection such as Cedara, the 50% growth reduction of F. verticillioides target DNA levels (1 335,2 pg µg-1, 1 140,5 pg µg-1 and 1 123,4 pg µg-1) is still relatively high and can be considered as a yield constraint. Therefore, the reduction of fungal infection by certain fungicides should be seen as an added advantage, but should be used in an integrated pest management system to mitigate maize ear rot infections and their related mycotoxins. It is reported in literature that the stress of fungicidal treatments can affect other facets of fungal metabolism, including sporulation and secondary metabolite (mycotoxin) accumulation. In this study elevated mycotoxin levels were not recorded for fungicide spray combinations and this will be closely monitored in the second year data that will follow.



















