ARC-Grain Crops,
Potchefstroom
In South Africa, maize eyespot has generally been of minor economic importance due to its limited incidence range (spatial) and low disease severities, with insignificant yield losses reported even
in more severely affected areas.
Originally this disease, caused by the fungus Aureobasidium zeae (synonym: Kabatiella zeae), appeared in the cool, humid areas of the south-eastern Free State, northern KwaZulu-Natal and south-eastern Mpumalanga in the 1992/1993 season. Initially, the disease was found in a few fields and deemed insignificant. During the 1993/1994 season the disease was more widespread and disease severity was higher, particularly in the Heidelberg district. Infection levels were so high that the leaves turned yellow and were full of small necrotic lesions. Symptoms then disappeared, likely due to a change in weather patterns where these areas were subjected to less humid, warmer weather.
During the current season, the disease was reported in the Viljoenskroon and Bothaville areas. Affected leaves received to date, however, showed low disease severity even though disease incidence in the fields is high. The outbreak of this disease in these districts is unexpected. The Viljoenskroon and Bothaville areas are normally associated with moderate rainfall and warm days in excess of 30°C, while this disease favours long periods of cool, wet weather during the growing season.
Symptoms
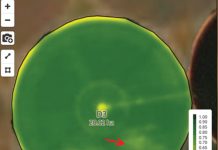
Symptoms of eyespot on maize generally appear two to four weeks before flowering and are most severe on the upper leaves of plants approaching maturity. The upper leaves are necessary for grain fill, so one would expect minor yield losses when these upper leaves become severely infected. Initially, symptoms appear as small (1 mm to 2 mm in diameter) circular to oval lesions that are cream to brown in colour and surrounded by a narrow halo (Photo 1). These lesions can coalesce to form larger necrotic regions on the leaf.

To isolate the fungus from leaf lesions, pathologists cut lesions into 1 mm to 2 mm squares and then surface sterilise them in commercial bleach for one minute. These leaf pieces are then incubated on potato dextrose agar at 26°C in darkness. Colonies are slow growing and initially colourless, but become pink with a leathery appearance after seven days (Photo 2). After twelve days the colonies become dark grey to black. Pure representative cultures of A. zeae grow best on 4% malt agar, and can be described to species level using monographs and identification keys. Aureobasidium zeae produce falcate, single-celled, extremely small hyaline conidia.

Epidemiology
The most frequently reported source of eyespot infection is crop debris left over from the previous season or where monoculture maize is produced. A. zeae overwinters as stromata, which are formed on infected maize plants at the end of the maize growing season. During spring the stromata produce conidia, which are then dispersed by wind or light rain to the leaves of nearby young maize plants where they germinate.
Maize seedlings are most susceptible at 10°C to 12°C. Secondary spread of the eyespot disease occurs through transferring of spores by wind and water splash from one plant to another. The incubation period for the disease is about four to ten days, depending on weather conditions. The production of conidia and the development of leaf eyespot are favoured by long periods of cool, wet weather. Regions with cool, moist environments are, consequently, most affected by the disease.
Eyespot disease may also be seed borne, although there is contradictory information on whether it is or not. However, this source of inoculum is negligible compared to the number of spores produced on infested crop residues. It was suggested that levels of A. zeae were detected in 2% of seeds from ears inoculated under the husk. There are reports implicating that A. zeae entered the United Kingdom via imported maize seeds. The possibility of the pathogen being seed borne needs further study to verify if this is the case or not.
Control
Cultivation and crop rotation
Although cases of the disease have been encountered on cultivated fields and on fields where maize has never been grown, careful cultivation and crop rotation can help to reduce early infection. The recommended system encompasses the use of a three- to four-year interval for maize cultivation in the same field. However, this is impractical in South Africa where maize is the primary crop grown with various other crops being used in rotation.
Due to the economics of maize production, many South African producers still insist on planting monoculture maize, which will be favourable to disease development. The amount of infectious material can be reduced by a suitable crop rotation with non-hosts and thorough ploughing and destruction of after-harvest residues, particularly that of seriously infected plants. Deep ploughing of crop debris prevents sporulation of the stromata and promotes decomposition, which limits early season spread.
Host plant resistance
Host plant resistance to A. zeae is important in the control of eyespot and hybrids with some resistance to the disease are recommended. However, South African maize hybrids have not been screened specifically for maize eyespot due to the low and periodic outbreaks of the disease.
Chemical control
This disease is rarely a target for foliar fungicides internationally, where the disease occurs endemically but severities are very low. However, fungicides registered for use against eyespot include mancozeb, propiconazole, chlorothalonil and benomyl, which can be used when the disease becomes an epidemic and severities are very high. Some researchers have suggested that seed dressings can give effective protection against A. zeae. Should the disease epidemic still develop, spraying the plants at the early stages of disease development – when 1% or less of the leaf area is infected – would reduce infections. More than one application may be necessary when conditions are favourable to disease development.
The use of fungicides against eyespot can be prohibitively expensive, except on seed production fields. From the literature and various points of view discussed, the role of fungicides has not been adequately researched, appearing to contradict one another. It is obvious that the role of fungicide control for eyespot still requires further studies to confirm the efficacy, economics and the time to spray.
To date the low levels of disease severity on plants have not warranted any fungicide spraying. Producers are, however, urged to monitor fields that are showing infections regularly and approach their local plant pathologist or send samples to the ARC-Grain Crops for further advice.
 When inspecting fields for eyespot, look for typical symptoms on the leaves as described below. The degree of infection can be estimated by determining the leaf area affected, using a five-degree scale where: 1° = 0% to 1,5%; 2° = 5% to 15%; 3° = 15% to 30%; 4° = 30% to 50% and 5° = >50%. Should the cool, wet weather persist for a second season, disease severities may increase similar to that observed during the epidemic of the mid-90s. Under these extreme conditions, yield losses may persist as recorded in the United States, New Zealand, Japan and Germany.
When inspecting fields for eyespot, look for typical symptoms on the leaves as described below. The degree of infection can be estimated by determining the leaf area affected, using a five-degree scale where: 1° = 0% to 1,5%; 2° = 5% to 15%; 3° = 15% to 30%; 4° = 30% to 50% and 5° = >50%. Should the cool, wet weather persist for a second season, disease severities may increase similar to that observed during the epidemic of the mid-90s. Under these extreme conditions, yield losses may persist as recorded in the United States, New Zealand, Japan and Germany.