
Five fungal genera have been primarily identified as the causal agents of seedling and/or root rots in dry bean production in South Africa. The most common fungi of these genera include Fusarium spp., Pythium spp., Rhizoctonia solani and Macrophomina phaseolina (charcoal rot). The last three pathogens may potentially prevent seed germination, which is a result of damping-off of seedlings and rotting of the seed in the soil.
Pathogens associated with root rot and seedling blight often appear as a complex consisting of more than one pathogen, which complicates the identification of the primary causal agent. Seedling blight and root rots are generally more prevalent in wet soils while Fusarium spp. are most common and problematic in drier years. The interaction of root rot diseases with cultural factors such as soil temperature, soil pH, fertility (such as soil nutrient concentration) and bean cultivars is often confounded with the prevailing environmental conditions and, in many cases, with other root rot diseases. These diseases kill or weaken seedlings, manifesting as patches of missing plants in a field that directly reduces pod numbers, yield, and, subsequently, economic viability of dry bean fields. The high risk of seedling and root rot development often prompt growers to use shorter duration seed and plant more seed per area.
Disease cycle and epidemiology
These seed- and soil-borne fungi (depending on prevailing climatic conditions) can infect the plant at any growth stage from germination up to maturity. The fungi may infect any part of the root system and can also occur just above the soil surface.
Many cultivated soils in the historic dry bean production area are infested by Fusarium spp. root-rot fungi. Therefore, most plants grown in these soils become partially or completely infected before they reach maturity. Inoculum is carried by wind, irrigation water, and equipment while seed lots can be contaminated by infested soil. In the absence of a dry bean crop between two growing seasons, the pathogen might persist on infected crop debris. The pathogen is also capable of colonising organic matter and roots of non-host plants without causing disease symptoms. This way it can maintain or increase its population in the absence of dry beans. The effect of Fusarium root rot is most noticeable during flowering and early pod set, when the plant and its productivity are more sensitive to stress.
Pythium spp. are common soil-borne plant disease pathogens that can survive as spores present in the soil and on plant debris. In the soil this pathogen can survive for several months or even a year. While Pythium infections can happen at any time of the season, older plants are more susceptible to infection when the weather is particularly conducive and under moist conditions. Infections cause a reduction and discolouration of the root system and complete rotting and decay of fibrous rootlets. Under continuous moist conditions, the pathogen spreads to infect the whole plant, resulting in wilting and plant death.
Rhizoctonia is a seed-borne pathogen and disease, and severity is influenced by soil moisture, soil temperature, nutritional status, and the plant and root exudates. Disease is more severe during the first two to three weeks under wet conditions and somewhat cool weather. The pathogen survives in the soil and on plant debris. Optimal soil temperature for the development of hypocotyl canker is 18 ºC. Relatively few cankers develop below 12 ºC.
The fungus that causes charcoal rot (M. phaseolina), thrives in soil and plant debris (where it can also colonise and then remain in a resting state) in the form of tiny, rigid, black structures throughout the season. On damp soils, it is harmful to young seedlings. After the fungus infects the roots, it gradually spreads through the root and stem tissue. Early-season infection symptoms start to appear from the middle of the season onwards, especially when the plants are under stress. The onset (particularly when beans are in their reproductive growth stages) and duration of these stress conditions that include intense heat and drought conditions, will affect the kind and intensity of symptoms that manifest.
Symptoms and occurrence
The occurrence of root rot diseases depends on environmental conditions, emphasising the need for a better understanding of the interaction between the seedling, root rots and environmental factors. With this knowledge, one can identify the conditions favourable for these diseases and predict when seedling and root rot diseases may occur.
Fusarium root rot, caused by several Fusarium spp. and associated with other root rot diseases, is usually of minor importance, although it appears to be the most common of the root rot diseases. However, during the last four seasons, it became a major dry bean disease in South Africa where it can often take out whole dry bean fields. These serious field infections often confound the assessment of the other root rot diseases. In contrast to other root-rotting diseases, Fusarium root rot does not generally cause seed rot or seedling damping-off in the early growth stages. Symptoms appear two to three weeks after planting, initially as narrow, lengthy, reddish-brown lesions on the stems, frequently with initial symptoms of lengthwise cracks appearing on the stalk. Lesions may affect the primary taproot in such a way that may cause it to shrink, degrade, or die. Above the shrivelled taproot, typical fibrous root clusters that are also known as adventitious or lateral roots, form. Under the best growing circumstances these roots can partially make up for dead roots and reduce symptoms visible above ground. Above the ground, infected plants are light green to yellow in colour, stunted and have a slower growth rate compared to that of healthy plants (Photo 1 – 4).


Pythium spp. in bean seed cause radicle and hypocotyl tissues to discolour and become soft and mushy due to seed rot and pre-emergence damping-off. Affected seedlings are unable to emerge from the soil. When post-emergence damping-off occurs, the roots and the hypocotyl develop water-soaked lesions below the soil surface, causing seedlings to wilt and die after emergence, thereby diminishing plant stands. Surviving seedlings frequently exhibit stunted growth and tiny, necrotic root systems. Initial symptoms on infected plants appear as dark-brown, water-soaked lesions on the lower stem surface area just below the soil line. These lesions extend downward through stem tissue into the taproot. Root-rot symptoms start when the cortical tissue is destroyed. Under moist conditions, lesions on the stem tissue will continue to progress downward and may eventually kill the entire root system. Above the ground, the plant will turn yellow and wilt. Plant yield, however, can be affected negatively by mild feeder-root infections even in the absence of aboveground symptoms (Photo 5 – 6).

(Photos: HF Schwartz)
Rhizoctonia root rot, caused by the fungus Rhizoctonia solani, is less commonly observed but often associated with other root rot diseases. For example, Rhizoctonia root rot can affect plant stands of plants that initially survived Fusarium root dieback stages to further increase yield losses. R. solani may induce seed rot, damping-off, stem canker, root rot and pod rot. When infections occur before germination, seed will decay. Lesions on a young seedling expand rapidly and results in damping-off. Seeds and seedling infections affect seedling establishment, which result in lower plant densities that can often be visually observed. The characteristic symptoms of Rhizoctonia root rot on infected plants manifest as reddish brown, sunken lesions on the stem. As infections progress, sunken cankers enlarge and may coalesce to girdle the stem, retard growth and, eventually, kill the plant (Photo 7 – 8 and Photo 9).

(Photo: Edward Sikora, Auburn University, Bugwood.org)

(Photo: Sirel Ozan Canpolat)

(HF Schwartz, Colorado State University, Bugwood.org)
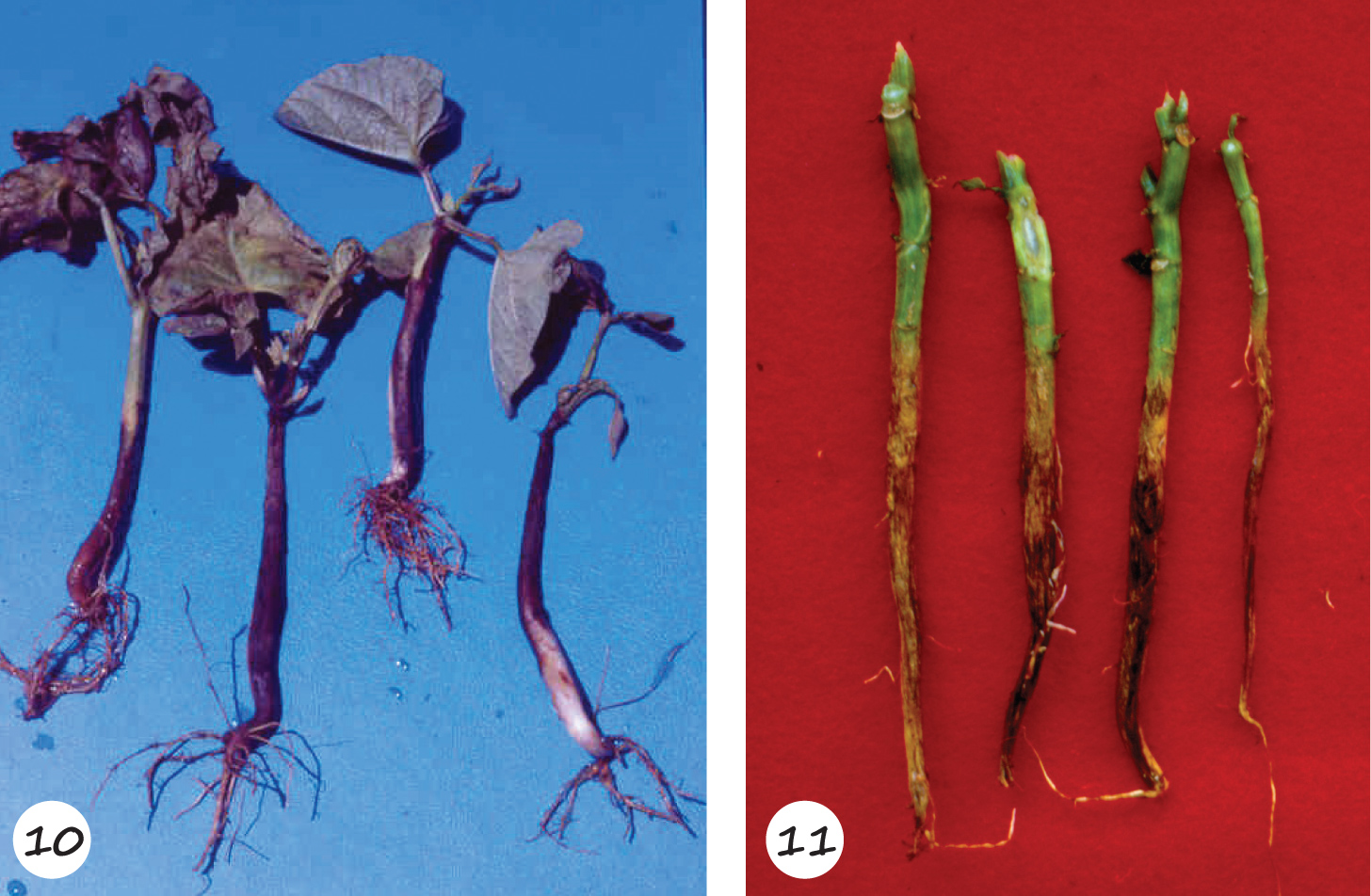
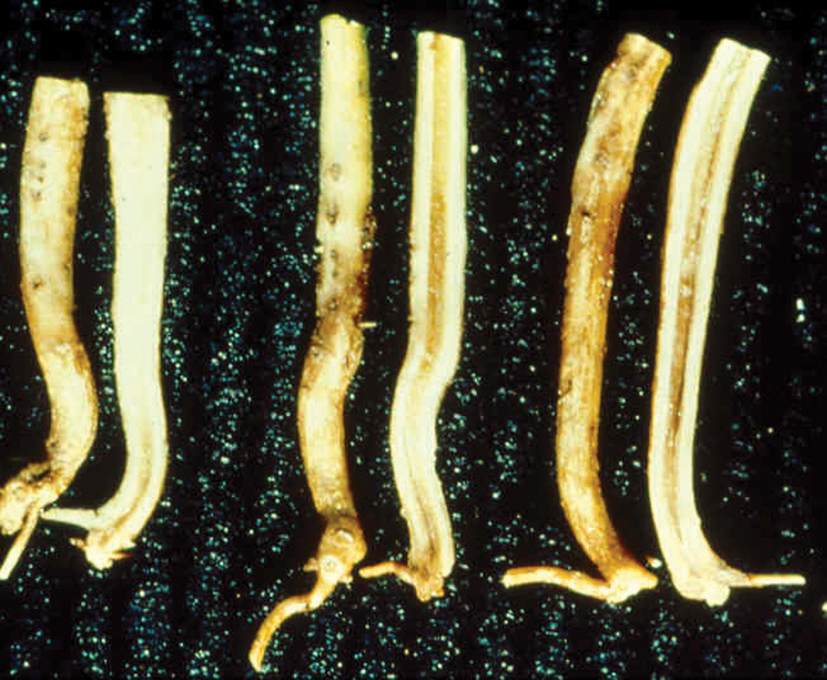
Charcoal rot, caused by M. phaseolina that survives in soil and host crop debris, may affect emergence, or discolour seedlings that can eventually die off when infected during seed or seedling stages. Symptoms appear as brown to dark patches on the cotyledons after emergence within two to three weeks, and round to rectangular, reddish-brown lesions on the growing hypocotyls from the unifoliate leaf stage onward. These lesions may turn dark brown or black over several days. Under hot and dry conditions, infected seedlings can die off. On more mature plants these lesions proceed up the stem and result in stem rot. It may also result in wilting, yellowing and decreased vigour. These symptoms are typically the first sign of M. phaseolina infection. Premature death with leaves still connected to the plant is characteristic of charcoal rot
(Photo 10 – 11).
Disease management
Control of seedling blights and root rots once present is problematic as this disease is only noticed once poor germination in the case of seedling blight and yellowing of the plants in the case of stalk rots occur. This accentuates the need for regular monitoring of fields throughout the dry bean-growing season to determine and assess the impact of seedling blight and stalk rots based on the symptoms described above.
While not treatable once present, root rot can be somewhat avoided through the application of preventative measures where these diseases have been recorded to occur. Integrated disease management utilising resistant varieties, fungicide treatments and appropriate cultural practices can control and reduce seedling and root rot of beans. The use of resistant varieties in combination with an effective integrated control programme will provide growers with added protection from seedling and root rot losses.

Host resistance
Planting resistant dry bean cultivars and varieties in fields with a history of the disease will reduce yield losses. However, the possibility of resistance breakdown due to pathogen adaptability and the development or emergence of new races of the pathogen may render previously resistant hybrids susceptible and should therefore be monitored.
Cultural control
Planting beans on coarse, well-drained soil appropriately fertilised at a depth of half an inch after soils have warmed up to 12 ºC, is the best preventive measure. A well-prepared seedbed reduces root rot and encourages quick seedling germination and growth. Reducing soil compaction and avoidance or breaking of hardpans are crucial because physical factors can significantly restrict root growth and reduce oxygen supply to the roots, hence exacerbating the plant’s stress levels. Control soil moisture with plant spacing and irrigation to provide growing plants with optimal water requirements without overwatering, because stress caused by flooding or drought renders plants more susceptible to infection.
Utilise a lengthy rotation (more than three years) of beans interplanted with cover crops of grass or cereal and non-host crops like maize or wheat. Keep in mind that each pathogen has its own optimum conditions for infection and disease development. Sometimes the primary infection by the root and stalk rot pathogens discussed may be followed by secondary infections of other diseases which are often less pathogenic but are able to seize the infection opportunity created to them by the primary pathogens.
Biological control
Increasing the slope and water-holding capacity of the soil and incorporation of organic matter may help prevent seedling and root rot. Natural biocontrol fungicide species such as Trichoderma spp. possess multiple mechanisms of action by which they control plant pathogens and promote plant growth. However, before using biocontrol agents producers should seek efficacy data from the relevant companies that market such products. Using ineffective biocontrol chemicals can be costly and result in economic losses.
Chemical control
Seed treatment with effective fungicides, especially when applied as a slurry, will protect against seed root and seedling damping-off in an infested field and thus ensure good seedling establishment. Fungicide treatments such as benomyl, mancozeb and thiram have been proven to be effective but seldom provide protection to the expanding root zone of older plants. It is therefore ineffective for controlling the root rot phase of the pathogen, particularly on plants past the seedling stage. Certain herbicides have been reported to both increase and decrease root rot severity.
 Dry bean producers are urged to communicate with their nearest plant pathologist for assistance in developing an integrated disease management system custom made to their specific needs. They are advised to contact the author at 018 299 6100 at the ARC-Grain Crops in Potchefstroom for any advice. Additionally, the researchers would like to request producers to report any disease symptoms observed so that it could be arranged to sample the causal isolates. Such samples are essential for research purposes and the assistance of producers in this regard will be highly appreciated.
Dry bean producers are urged to communicate with their nearest plant pathologist for assistance in developing an integrated disease management system custom made to their specific needs. They are advised to contact the author at 018 299 6100 at the ARC-Grain Crops in Potchefstroom for any advice. Additionally, the researchers would like to request producers to report any disease symptoms observed so that it could be arranged to sample the causal isolates. Such samples are essential for research purposes and the assistance of producers in this regard will be highly appreciated.
This article was co-authored by the late Dr Bradley Flett.




























