ARC-Grain Crops, Potchefstroom

Dr Belinda Janse
van Rensburg,
ARC-Grain Crops, Potchefstroom
Sclerotium rolfsii (Athelia rolfsii) commonly known as southern blight, southern stem blight or white mould, infects more than 500 plant species including crops such as groundnut, sunflower, soybean, potato and tobacco to name a few. Although certain graminaceous crops such as wheat, millet, grain sorghum and other grasses may also be infected by this fungus, it is uncommon.
The disease occurs worldwide, but is more prevalent in warmer (above 24 oC), moist climates. During the current growing season, the ARC-Grain Crops have been inundated with queries regarding this disease on soybean, groundnut and sunflower.
In Potchefstroom, sunflowers at the V4 growth stage were more susceptible to infection when compared to R5.1 sunflower in an adjacent field. S. rolfsii normally infects the lower stem of the plant near the soil surface interface. In some plants it may infect the roots as well. On low-growing plants such as groundnut and soybean, leaves touching the soil may also be infected. S. rolfsii-infected sunflower plants in Potchefstroom exhibited root and crown rot and the dieback of several plants within a plant row (Photo 1). This article aims to give producers more information regarding this disease and its control options, which remain difficult due to the pathogen’s wide host range.

Disease symptoms
On sunflower (where the disease is often referred to as collar rot), groundnut and soybean the disease starts with a small water-soaked lesion on the lower stem at the soil surface. This lesion spreads around and finally girdles the stem of the host plant, resulting in the wilting of the plant. The wilted leaves generally turn yellow and then brown, but usually remain hanging on the plant.
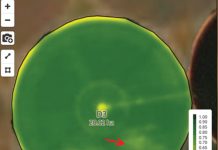
As the lower infected portions of the stem are infected and begin to decay, a white mat of mycelium develops around the lesion site. This mycelial mass often spreads out onto the nearby soil surface in a fan-shaped fashion. Round, white sclerotia of 0,5 to 1 mm will develop (Photo 2). In dry weather, strands or fans of mycelium are less apparent and, instead, white to pale buff mycelia are formed that look like small patches of paint appressed to lower stems. These mycelia then become smooth, round structures and change colour to light tan, brown or black. Sclerotia are overwintering structures and can be seen in the mycelia or on diseased tissues just above or below the ground surface. The foliage begins to wilt, and dieback occurs due to rotting of the stem tissue (Photo 3). In soybeans, lower leaves generally develop brown spots which expand and eventually turn the foliage brown, even though they remain attached to the stem.


Biology of the pathogen
Many fungi have both asexual and sexual reproductive stages. Each stage has been given a different name. Thus, two different names have often been given for the same fungus. The logic behind this is unclear and there are moves to give each fungal species a single name.
This pathogen has an asexual stage referred to as Sclerotium rolfsii which produces the typical symptoms mentioned above. Mature sclerotia consist of an outer thickened and tough mycelial rind which surrounds a layer of thin-walled mycelia. Sclerotia develop to 0,5 mm to 2 mm in diameter, but some may be as large as 10 mm. Hyphal segments may also serve as inoculum sources and can overwinter in plant debris or as sclerotia. Sclerotia have been shown to survive for up to three to four years in soil. S. rolfsii doesn’t produce any asexual spores.
The sexual stage of this fungus is referred to as Athelia rolfsii, but it is not frequently seen. Typical of the basidiomycetes, A. rolfsii produces basidiospores attached to a basidium and this is when genetic exchange occurs. These basidiospores are the multicyclic stage of the pathogen’s life cycle and are released into the air. When in contact with susceptible host tissue, the spores swell and produce germ tubes that produce appressoria (infection pegs), penetrating the host tissue.
Disease cycle and epidemiology
At the onset of planting and when favourable warm wet conditions prevail, the sclerotia germinate and develop hyphae. The hyphae infect plant material of any susceptible hosts within their wide host range. S. rolfsii is a necrotrophic, soil-borne pathogen that thrives in highly aerobic environments and therefore survives best on or near the soil surface. Wounds caused to plants by hoeing, mechanical machinery moving through the land, nematodes or insect damage increase the possibility of fungal infections. Once infection occurs the fungus produces oxalic acid and specific enzymes which cause death of host plant cells and facilitate fungal growth and infection. The fungus overwinters as sclerotia and mycelia in infected host tissue (Photo 4). Sclerotia have been known to survive several years in the absence of a host.

This disease is a major problem in warm and wet tropical and subtropical areas. Both temperature and moisture are significant factors in the spread and development of the disease. Optimal growth and Sclerotinia formation occur between 25 oC and 35 oC. However, sclerotia have been reported to survive in locations where below freezing temperatures occur. Germination of sclerotia is stimulated by the presence of volatile, organic compounds released by decaying plant debris. Current season spread occurs by mycelial growth from debris, organic matter or sclerotia. This fungus does not produce spores that move in the air, so the disease is confined to localised areas in a field where sclerotia reside. Under favourable conditions, the disease spreads by plant-to-plant contact and infection. Infected plants are often found to occur within a row where plant-to-plant contact occurs. Movement of infected material or infested soil results in long-distance spread of the disease.
Groundnut and soybean
Groundnut and soybean are infected by sclerotia or hyphae under favourable conditions. These infect the stem near the soil surface, producing silky white mycelium on the stem. As the fungus further colonises tissues of the lower stem, yellowing of the plant, wilting and death follow (Photo 5). This results in yield and quality losses. On infected groundnut plants one or two branches may die off while few branches may still survive. Groundnut or soybean infected during dry weather have small cankers associated with infection and very seldom wilt. However, if infected during wet, hot weather, stems generally become totally rotted and the plant wilts and dies. Peg and pod infections are also common and result in pod loss. Rotted pods are thin and brittle and the kernels may be stained, rotted or absent.

Sunflower
Under warm, humid and wet conditions the incidence and severity of collar rot increase. Yield losses of 10% to 15% have been recorded worldwide, but the disease is less prevalent in South Africa. Typical symptoms are rapid wilting of plants with a brownish lesion that girdles the stem base near the soil. As in soybean and groundnut, the white mycelium forms on infected tissue, often radiates over the soil surface and produces small, brown, spherical sclerotia. Cycles of wet and drying have been reported to stimulate germination of sclerotia.
Control
Control of this pathogen is difficult as it produces sclerotia which overwinter in the soil to emerge as inoculum and cause disease the following season. Various chemical, biological and cultural control strategies have been suggested and implemented, some of which have reduced disease incidence in the field. No groundnut cultivar is completely resistant to infection by S. rolfsii and disease management is extremely challenging.
Cultural practices
Soil tillage, prior to planting groundnut, bury sclerotia and thus reduce inoculum levels. Generally, a mouldboard plough will bury inoculum deep enough. However, with the change to minimum tillage which became more economical and convenient for some producers, this is not always a practical option. Various researchers have had different results regarding the effect of tillage on this disease. This difference of opinions and results may be due to factors including soil type, depth of ploughing and microclimate variations. Sclerotial germination has been found to be lower at depths below 2,5 cm compared to that at the soil surface and does not occur at depths of 8 cm or more.
Deep ploughing has thus been suggested as a means of cultural control because survival of the sclerotia of this high-oxygen-demanding fungus is a year or less when buried deeply. Research done at Viljoenskroon on sandy soils showed that ploughing deep using an inversion plough reduced infection levels. These sandy soils are, however, very prone to erosion when left with no crop cover and the producer has to consider the advantages and disadvantages when deciding on a ploughing method. Cultivation during the season should be avoided as any mechanical stem damage may increase chances of infection. The aeration of the soil allows deeper penetration of the fungus where roots, pegs and pods can be infected. Cultivation during the season can, furthermore, promote disease by uncovering buried sclerotia, improving aeration, and by throwing infested soil onto plants.
Irrigation may be timed to prevent excessive moisture during warm periods, which will increase infections. Producers should allow a dry period between irrigation schedules to reduce infections as prolonged periods of wetness below the canopy will favour disease development. Various studies indicated that increased canopy closure increased infection levels.
Reduced plant densities in a South African study conducted at Viljoenskroon (sandy soils) increased the incidence of disease in an infected field. Therefore, it is not considered to be a viable form of cultural control on these soils. The Viljoenskroon groundnut-producing area in which this trial was conducted, is notorious for wind erosion. The lower planting densities were more wind-prone, causing wind-borne sand to damage the plants or to heap up around the stems of the plants and cover the runner stems, which increased the possibility of infection. It would therefore appear that although soil-borne disease severity may be stimulated by lush foliar growth of the canopy, a decrease in the plant density is not a viable option for reducing S. rolfsii damage in areas that are prone to wind erosion.
Crop rotation is effective if producers ensure that the crops rotated are not hosts of the fungus – it has a very wide host range. Maize, grain sorghum, small grains, Sudan grass and cotton are possible alternatives. However, some researchers have claimed that cotton and grain sorghum may also be hosts of the pathogen. A minimum of two-year rotations is required where the disease pressure is known to be high. Rotations of two years or more to a non-host crop help to prevent build-up of inoculum and disease problems in groundnut. Longer rotations are necessary to reduce disease once high levels of inoculum are established. Weed control is very important and needs to be maintained to prevent disease increase on susceptible weed species, which include mainly dicotyledonous and a few families of monocotyledonous plant species.
Planting dates have been shown to influence the incidence and severity of collar rot on sunflower and may be manipulated to avoid high infestation times. However, this is not always practical as one requires some sort of accurate weather prediction to avoid warm, humid, wet conditions.
Chemical control using fungicides
Fields with a history of the disease should be scouted weekly to determine onset of this disease. Where the disease is known to be a problem, fungicides may be used to control the disease to a certain extent. In the USA several effective fungicides are registered for use on groundnuts. Most of these fungicides also control leaf spot, which makes their use economical. In a study done in South Africa and published in 2003, Difenoconazole in combination with a Trichoderma harzianum biocontrol agent reduced the growth rate of S. rolfsii. Since then, many new products have appeared on the market that may be better chemical control possibilities. However, they need to be tested.
Fungicide efficacy depends on canopy penetration and ensuring that the fungicide reaches the target. At times two sprays may be necessary, however, producers need to consider the economics of chemical control. In the USA, spray programmes may be as high as four spraying intervals that start when the groundnut rows begin to close about 70 days after planting and thereafter applied at 14-day intervals. Locally, this would be costly and very high disease pressures need to be noted before going to such extremes.
 Resistance
Resistance
Work done in the USA showed that complete resistance to S. rolfsii in groundnut and soybean is not known. However, there is a limited number of cultivars with partial resistance that may be used in an integrated disease management system. In South Africa, no work has yet been done on resistance to this disease, but should the disease persist or get worse in the following seasons, local hybrids should be screened for resistance. Soybean is susceptible from emergence through pod fill, but producers should only be concerned when infections occur during the vegetative stages of growth.
For more information, contact Drs Bradley Flett and Belinda Janse van Rensburg at 018 299 6100.