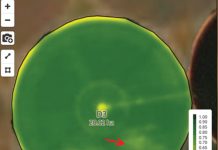
There are several Agrotis cutworm species (Lepidoptera: Noctuidae) present in South Africa, namely black cutworm (Agrotis ipsilon), grey cutworm (Agrotis subalba), brown cutworm (Agrotis longidentifera), spiny cutworm (Agrotis spinifera) and the common cutworm (Agrotis segetum). The common cutworm is the most prominent and economically important species present in South Africa. The larvae (Photo 1) are dirty-grey or brown in colour with a smooth, waxy appearance.

Photo: http://www.pyrgus.de/Agrotis_segetum_en.html.
Why it is important to get cutworm management right
- Larval feeding can result in severe damage to crops during the seedling stage.
- The larvae move from one seedling to another, cutting and destroying the stems of seedlings close to ground level (Photo 2), often resulting in death.
- One larva can damage numerous plants in a single night.
- If outbreaks occur, replanting of the crop often has to be done.
- These larvae are active at night and during the day they can be found close to the soil surface near dead seedlings.
- Damage resulting from cutworm is not only restricted to seedlings. Plants at the four-leaf stage or older may also be damaged.
- This damage in older plants can be identified as round holes into the stem, just below the soil surface.

Photo: Clemson University – USDA Cooperative Extension Slide Series, Bugwood.org
Why producers need to scout
- Scouting constitutes one of the best weapons producers have in their arsenal to combat cutworm.
- Emerging crop seedlings must be continuously inspected for signs of cutworm, preferably twice per week, and treated when necessary.
- Scouting post spraying is vital to determine if the initial application was successful or if a second application (depending on the label of a product) is required.
- Producers who apply insecticides at planting must also scout to determine if control has been achieved.
- The edges around bare regions should be inspected by producers for recently ‘cut’ plants. In addition, the top 5 cm of soil should be thoroughly searched for larvae.
- Where any notched, wilted, dead/cut weed or crop seedlings are observed, one should start digging around roots of the plants to identify cutworm larvae.
Management strategies
Genetically modified maize (Bt)
While genetically modified (Bt) maize is effective against stalk borers, it is ineffective when it comes to cutworm. The reason is simple – cutworm and stalk borers are different species. Cry proteins in Bt maize are species specific. In addition, as with insecticides, the size of the larvae being treated is crucial. Therefore, the bigger the larvae, the less effective the cry proteins. Cutworm larvae that target maize seedlings are generally large, late-instar larvae (Photos 1 and 2). Producers must be vigilant of any seed companies claiming that the Bt gene will control cutworm larvae – this is patently false based on the reasons highlighted above, as well as the fact that there are no registrations under Act No. 36 of 1947 in this regard.
Controlling weeds – why is it necessary?
Weed control through the responsible application of registered herbicides according to label instructions prior to planting, is an effective method to manage cutworm larvae. The reason is that newly hatched cutworm larvae rely on weeds for a food source when crops are not available. In general, a minimum of 35 weed free days prior to planting (where practically possible) is required in order to starve larvae. While producers may not have weeds at the time of planting, the presence of weeds four to five weeks prior will exacerbate cutworm issues.
Producers who plant maize cultivars with herbicide tolerance, would likely have to wait for seedling emergence before applying an herbicide. This is also true for insecticides.
Insecticides and the resistance question regarding pyrethroids
Applications of suitably registered pyrethroids and organophosphates (according to label instructions) are considered effective in controlling cutworm larvae. However, during the 2020/2021 growing season, reports of extremely high infestation levels of cutworm were received from the Wasbank region in KwaZulu-Natal (KZN), where maize fields were planted under no-till. In addition, similar reports were also received from the Bothaville region in the Free State. Producers believed that cutworms had developed resistance against pyrethroids because the same lambda-cyhalothrin treatment strategy used effectively in previous seasons against cutworm, was no longer effective. However, Dr Gerhard Verdoorn, CropLife SA operations and stewardship manager, questioned this assumption. ‘I was not convinced, because it was a sudden widespread outbreak and it followed a period of very good rain after the prolonged drought we had,’ he said.
During the 2021/2022 growing season, media reports highlighted the serious damage cutworms had caused to maize, potato and other vegetable crop fields. Where producers again suspected resistance, entomologists agreed with Dr Verdoorn. It was found that the damage being witnessed was a result of cutworms changing their behaviour by remaining and foraging below the soil surface. The sudden behavioural change was attributed to the very wet conditions that prevailed across most of the summer rainfall areas in the country. Some producers had no choice but to replant much of their crops; whereas potato and vegetable producers who experienced significant damage during the advanced growth stages, were unable to replant.
After numerous discussions with producers, Dr Verdoorn came to realise that this issue was likely due to the application method and crop residues, together with the amount of rain received. In general, it is common practice for producers to do a chemical mow-down with glyphosate before planting where a pyrethroid (mostly lambda-cyhalothrin) is mixed in for cutworm control. Dr Verdoorn suspected that a pyrethroid mixed with glyphosate might not be a good option, since the molecules may possibly interact or damage each other. Given that the producers affected were practicing no-till, cutworms likely escaped any pyrethroid that would have passed through the crop residues onto the soil surface during the very wet weather conditions experienced in the summer rainfall regions.
‘The practice of mixing a pyrethroid and glyphosate, the crop residues, the wet soil and a lepidopteran explosion thus made life difficult for no-till producers and easy for the cutworms.’ He further provided an example of a producer who converted from no-till to tillage well before planting in order to clear out weeds prior to applying lambda-cyhalothrin not mixed with glyphosate. ‘The producer had virtually no damage, whereas his neighbours who continued with no-till and some with tilling, but who used the lambda-cyhalothrin and glyphosate mix, had up to 80% damage. I hypothesise that tilling has a certain effect on cutworms, and tank mixes may contribute to ineffective control,’ he said.
The impact of soil cultivation differs for various pest species. In this instance, cultivation removes the host plants, whereas for stalk borers, the result is direct injury. Thus, timely soil cultivation to remove weeds will have an effect on cutworms since the action removes the host plants, and therefore their food. A switch from no-till to minimum tillage in the form of ripping (as suggested by some) would have no effect on cutworm.
Cutworm resistance to certain pyrethroids has been a hot topic in pest management of late. What is becoming clear, is that cutworm resistance to pyrethroids does not seem to be the issue. It is also clear that producers practising conventional tillage are able to better manage cutworm with no reports to date. A switch from no-till to minimum tillage in the form of ripping is unlikely to have a profound effect. Therefore, it appears as if the problem lies with the crop residues preventing the spray mixture from reaching the larvae in sufficient amounts to bring about appreciable control. This then also leads one to question whether the practice of mixing lambda-cyhalothrin and glyphosate should be recommended in no-till operations. Unfortunately it is not as simple as just changing to cypermethrin or deltamethrin either because from a chemistry perspective, questions still remain:
- Do these molecules react differently in tank mixes?
- Is it a timing issue affecting the efficacy of the active ingredient?
- Is it irresponsible tank mixing where label instructions are not followed?
Given Dr Verdoorn’s vast experience in the field of organic chemistry, he attempted to put the puzzle pieces together based on current knowledge. He noted that glyphosate in its pure form is a glycine acid which may be used as pure acid in some glyphosate herbicides, but may also be used in potassium, sodium isopropylamine or various ammonium salt forms in the herbicide formulations. The specific species of glyphosate used changes the acidity or pH of the spray mixture, which in itself may affect the pyrethroid molecule.
‘Most pyrethroids do not tolerate low pH well and pure glyphosate in water drops the pH down to 1,9; that is practically lethal to a pyrethroid molecule,’ he added. There is also a strong possibility of forming binary complexes between a glyphosate molecule and a pyrethroid where the two molecules bind through strong Van der Waals bonds; this can effectively deactivate both molecules.
‘The glyphosate molecule may also interfere with the pyrethroid product’s formulation by destabilising emulsifying agents, thus rendering the spray mixture ineffective against target insects.’ He agreed that irresponsible tank mixing is a cause for concern: ‘Worst of all is where a real home brew with as many as seven products, including nutrients, are mixed in a spray tank. It is unfathomable why some think it wise to stoop to this level of alchemy and then complain that products do not work,’ he concluded.
Conclusion
Cutworms have been relatively well managed in the past, but as with everything in life, nothing remains constant forever. Given the current information available, it appears that the problems in managing cutworm outbreaks in no-till systems may either be due to (1) tank mixes; (2) crop residues limiting pyrethroid contact with cutworms foraging below the soil surface due to the wet conditions; (3) timing of weed control, or (4) a combination thereof. Empirical evidence is required to accurately pinpoint the problem(s).
Producers must also ensure that they are strictly adhering to label instructions without deviation in any manner whatsoever when applying a pyrethroid. The label of a pesticide product is the law in terms of Act No. 36 of 1947. At present, the Insecticide Resistance Action Committee (IRAC) believes that there are no resistance issues given the available information. However, the committee remains vigilant. Finally, producers are encouraged to keep records of pest experiences linked to climatic conditions in order to better anticipate and prepare for problems in following seasons.
Producers requiring further advice or information should reach out to CropLife SA at info@croplife.co.za.