
What if we could cure cancer? Use a pair of scissors to cut out that cancer-causing gene? Well, maybe we can.
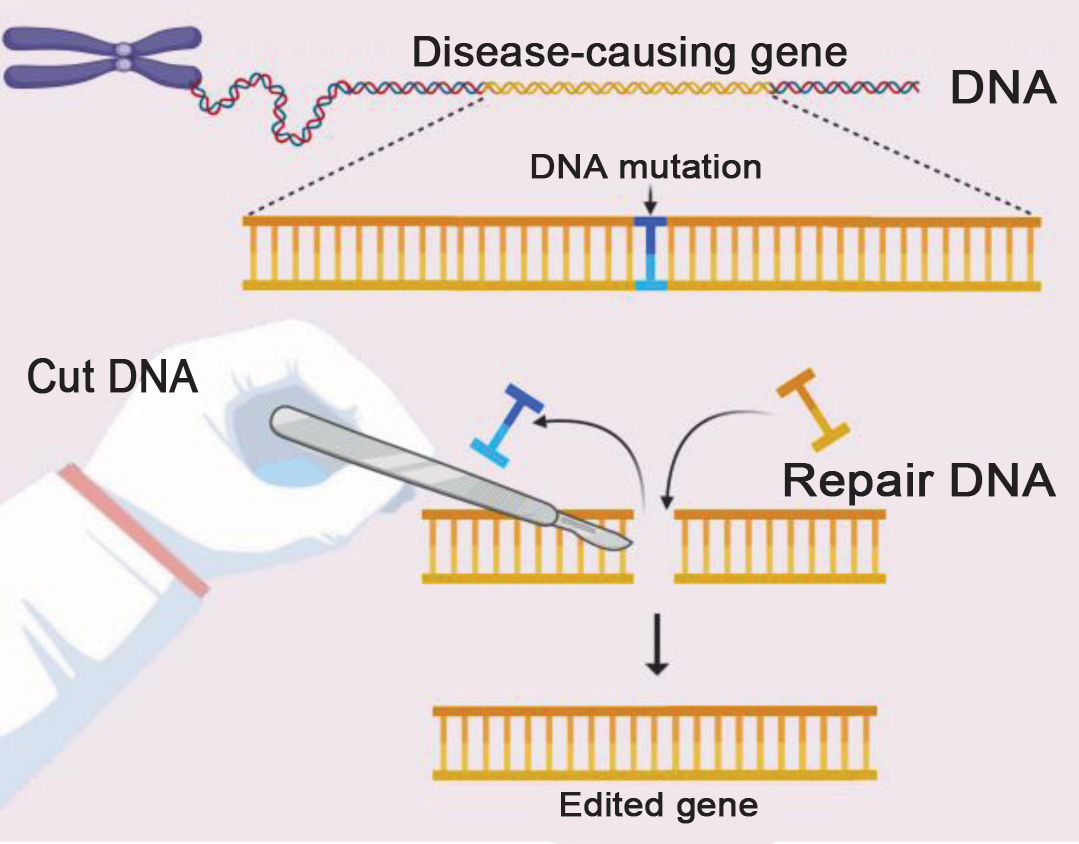
A genetic system called CRISPR-Cas9 was developed that allows us to edit gene sequences. The CRISPR-Cas9 system is derived from bacteria and uses the bacterium’s unique ability to make a cut in a gene sequence, which can be repaired in a few different ways. Firstly, it can leave it up to the host organism’s own machinery to repair. Secondly, it can make a specific replacement.
Genome editing
All our genetic information (DNA) is collectively called our genomes, and when we know where the problem is (such as the BRCA gene, which increases the odds for breast cancer), we can treat it. With CRISPR-Cas9, we could potentially change the unique sequence from cancer-causing to non-cancerous. It will still take many more years for us to easily and simply edit human genomes to prevent illnesses, but the potential is immense.
Our genomes are made up of genes, which are made up of building blocks called base pairs. Only four exist (A, C, G, and T), and the millions of possible ways these base pairs are arranged make not only species, but also individuals, unique.
Genome editing refers to a process wherein specific base pairs are changed (e.g. from A to T) to change the gene’s function. Genome sequencing determines how the base pairs are arranged, and this opened the door for determining gene function.
Source: Kato-Nitta, N, Maeda, T, Inagaki, Y et al. Expert and public perceptions of gene-edited crops: attitude changes in relation to scientific knowledge. Palgrave Commun 5, 137 (2019). https://doi.org/10.1057/s41599-019-0328-4
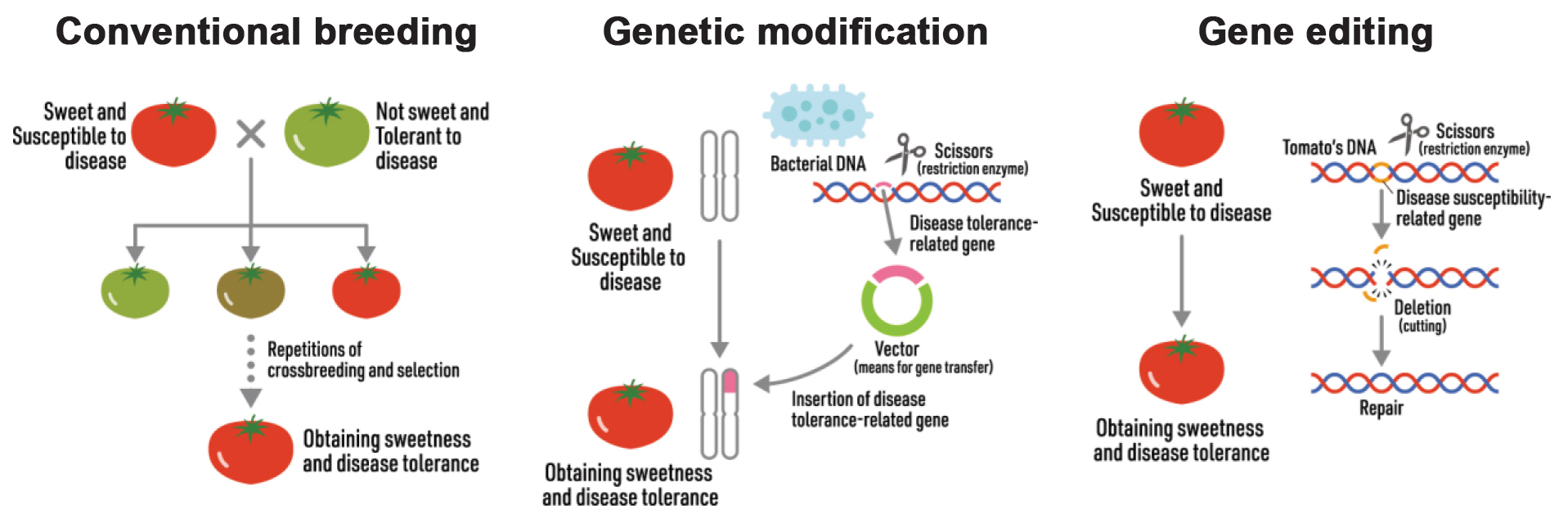
Genome editing versus genetic modification
Genetically modified organisms (GMOs) are different to genome-edited (or gene-edited) plants, since genetic modification entails transfer of a gene from one species to another. For example, resistance to the herbicide glyphosate is conferred to many crops (such as maize and soybean) via bacterial genes, and these crops are then referred to as Roundup Ready. Genome editing can be a short cut to conventional breeding, by targeting a gene within a specific host (thus no gene transfer from another species).
New breeding techniques
CRISPR-Cas9 is not the only genome editing system: a suite of similar systems exists that in the plant world is collectively called new breeding techniques (NBTs). These techniques are short cuts that decrease the time needed for conventional plant breeding. Very simply, genome editing works like ‘cut and replace’.
The world is excited about the prospect of using techniques that produce the same outcome as conventional breeding, but just much quicker.
Are all NBTs equal? No. In the broadest sense, new breeding techniques can deliver products that are i) indistinguishable from products obtained from conventional breeding (non-GMO); or ii) products that cannot be obtained via conventional breeding (i.e. can then be classified as GMO).
NBT crops
At the time of writing this article, we were not aware of any genome-edited grain crops produced using an NBT being grown commercially anywhere in the world. In Brazil, research into a drought-tolerant soybean variety is taking place. A gene was identified that senses water shortages and when shut off, makes the plant more tolerant to drought conditions. The yield may not be comparable to plants grown under ideal conditions but will fare better than the same plants under drought conditions.
Genome editing is expected to complement conventional breeding. While conventional breeding takes several years, it can be used to improve traits already in a gene pool, while genome editing can be used for specific and precise changes.
Risks
Regulations will still be put in place to monitor how new techniques and technologies are used. Across the world, countries are putting appropriate regulations in place to ensure responsible use of these new technologies.
Source: https://www.ucl.ac.uk/ioo/research/research-labs-and-groups/carr-lab/bestrophinopathies-resource-pages/research/research-3




























