
ARC-Small Grain,
Bethlehem
Weeds have a negative impact on any crop yield. Depending on the weed species present, such impact can range from very low to total crop loss.
Several studies have been conducted on the possible impact that weeds might have on crop production. In addition to antagonism as well as competition for soil nutrients and/or sunlight, some weeds are hardy and might interfere with the harvesting process if they are not controlled. The presence of some species of weed seeds in the crop harvest may also lead to downgrading of the crop, or the refusal of silos to even take in the delivery. Although a lot of research has been conducted on the influence of weeds on wheat growth, yield and quality, very little is known about the impact of weeds on barley.
Literature
Some research in a general agricultural production system in India found that weeds caused significant yield losses in barley and that the annual economic loss was substantial (Joshi, 2002). Chaudhary et al. (2008) reported that one kilogram of barley yield was lost with the presence of each kilogram of weed green biomass present. In an article published by Mahajan (2020), yield loss due to weed interference in eight barley genotypes ranged from 43% to 78%. Such huge losses can be attributed to the competitiveness of weeds with barley. The negative impact of individual weed species in cereals is determined by the combination of its features: period of germination, growth rate, size of the overhead mass, height and branching of stems, shape, size and position of leaves, levels of photosynthetic activity, ecological plasticity, coefficient of reproduction and others (Haigh, 2000). Bazitov et al. (2014) reported a significant increase in weed infestation in experimental areas of barley grown under irrigation. A similar scenario may also be applicable to irrigated barley in South Africa.
In South Africa, limited herbicides are available for use against weeds in barley. This leads to the repeated application of herbicides with the same mode of action, resulting in the development of resistant weeds. Although the presence of herbicide-resistant weeds in wheat has been studied extensively locally, the prevalence of herbicide-resistant weeds in barley remains unclear.
According to the Crop Estimates Committee (CEC), the production forecast for malting barley during the 2020/2021 season remained unchanged at 589 846 tons. The area planted was estimated at 141 690 ha, while the expected yield was 4,16 t/ha. This is the largest expected malting barley crop recorded for South Africa. The extensive area of barley planted necessitates weed management and the prevention of herbicide resistance. Due to the fact that barley is more sensitive to weed competition than wheat, early control measures are needed to realise optimal yield potential. The presence of uncontrolled, herbicide-resistant weeds might lead to larger shortfalls in barley production.
Research
Since 2013, ARC-Small Grain has been optimising the technical procedure of molecular screening for the identification of target-site herbicide resistance in ryegrass, but also other grass weed species. Since the same process can be tweaked for identification of target-site resistance in broad-leaved weeds, such weeds are also screened when received.
Target-site resistance is when a herbicide is unable to bind to the target site it was intended for, due to a DNA sequence change/mutation. It is therefore unable to inhibit that specific enzymatic/biochemical pathway and the plant survives (Figure 1 and Figure 2). Resistant weed biotypes, on average, require 10 to 30 times higher herbicide dosages than susceptible types. Importantly, molecular screening is optimised for detecting target-site resistance in Group A and Group B herbicides. These are also the herbicides that are predominantly used for post-emergence weed control in barley.
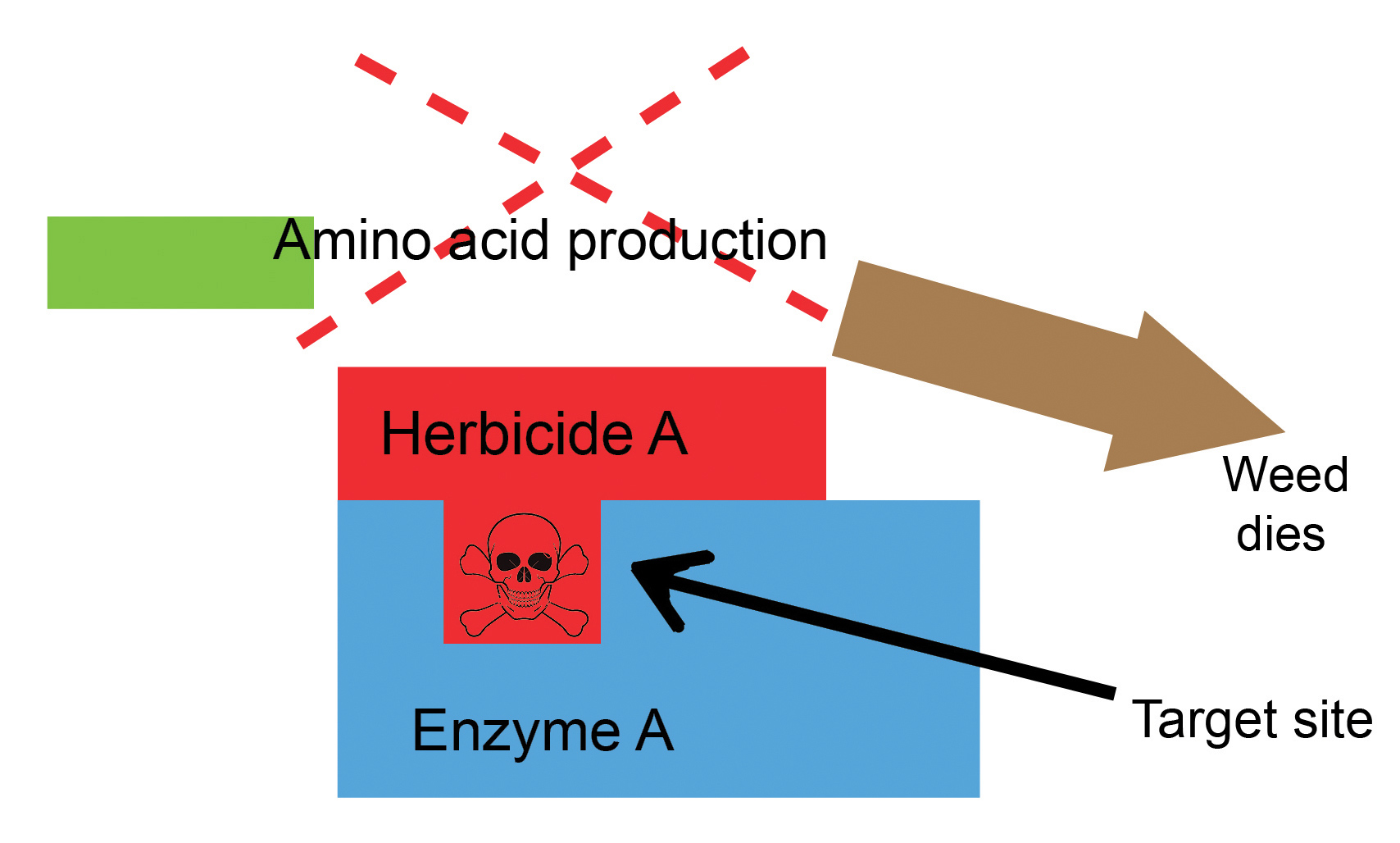
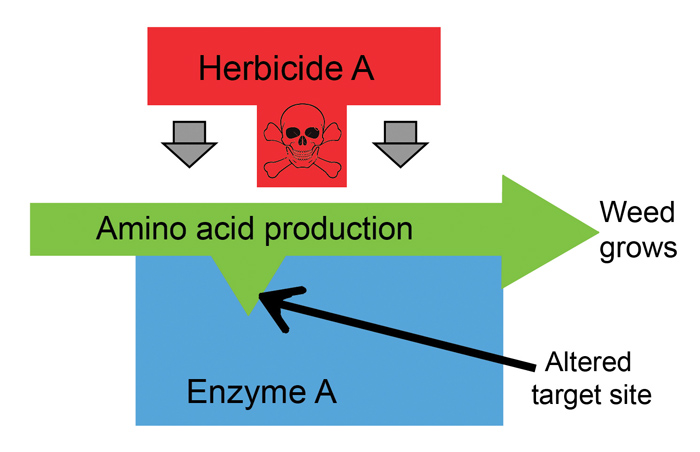
Results
From 2018 to 2021, a total of 90 samples were either received/collected from barley fields distributed throughout the Western Cape and summer rainfall irrigation areas. Some of the samples were screened for ALS resistance (Group B herbicides), but most of the samples were screened for ACCase resistance (Group A herbicides). As herbicides from the ACCase-inhibitor group are predominantly used, results are indicated in Graph 1.
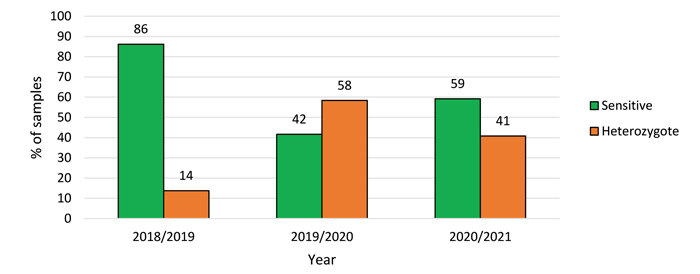
During the 2018/2019 season, 4 of 29 samples (14%) tested positive for target-site resistance to ACCase-inhibitor herbicides. During the 2019/2020 season, seven out of twelve samples (58%) tested positive for target-site resistance to ACCase-inhibitor herbicides and in the 2020/2021 season, 20 out of 49 samples (41%) tested positive for target-site resistance to herbicides from this group. Target-site resistance to the marker that was tested for ACCase target-site resistance, confers resistance to many aryloxyphenoxy-propionates (APP), all cyclohexanediones (CHD), including clethodim and phenylpyrazolines (PPZ). The following ACCase herbicides may show target-site resistance: clethodim, clodinafop, diclofop, fluazifop, haloxyfop, butroxydim, sethoxydim, tralkoxydim and pinoxaden. Heterozygous resistance means that there is a presence of target-site resistance in the tested sample, but that some degree of control can still be obtained with herbicides from the tested group. According to the results, more sensitive populations were recorded every year than heterozygous-resistant populations. It is, however, important to note that other mechanisms of resistance might occur in the sensitive populations. From Graph 1 it is also evident that during the last two years the number of heterozygous-resistant populations increased when compared to the 2018/2019 season. A possible reason might be that only problematic/surviving weeds were screened for target-site resistance.
How to get weeds tested for resistance
Producers are welcome to send grass weed seedlings or seeds to ARC-Small Grain (Blydskap Road S191, Bethlehem, 9700). Seedlings must be kept moist (preferably in Ziplock bags) and must be couriered if possible, as it will ensure that fresh seedlings arrive in Bethlehem. Please indicate the GPS coordinates where the plant or seed samples were taken. Seeds must be stored in brown paper bags to prevent them from being overrun by fungi or other contaminants. The seeds/seedlings must be a large enough bulk representative field sample to enable an accurate screening process. Please make sure that a number of samples from the problematic field are sent in; one plant only will not be adequately representative.
Summary
Target-site resistance may render some Group A (ACCase-inhibitor) herbicides less useful in the spraying programmes that are used during barley cultivation throughout South Africa. In cases where target-site resistance was confirmed, it is especially necessary to adopt additional methods of weed control. It remains important to be aware of the resistance status of the weeds in your crop, to be able to apply effective herbicide management strategies and achieve optimum barley yields.
References
- Chaudhary, SU, Hussain, M, Ali, MA & Iqbal, J. 2008. Effect of weed competition period on yield and yield components of wheat. Indian Journal of Agriculture research, 46:47 – 53.
- Joshi, NC. 2002. Manual of weed control. Research publication 7615-B, East Azad Nagar, Delhi-110 051.
- Mahajan, G, Hickey, L & Chauhan, BS. 2020. Response of barley genotypes to weed interference in Australia. Agronomy, 10(1).
- Bazitov, V & Bazitov, R. 2011. Influence of tillage and fertilization on weed infestation of barley grown in super intensive crop rotation. Sci Tech, 6:202 – 204.
- Haigh, T. 2000. Weed competition and control. Centre for Horticulture and Plant Sciences Technology and the Environment, University of Western Sydney.
For further information, contact Hestia Nienaber at 058 307 3420 or email deweth@arc.agric.za.
This research is made possible through financial assistance by AB InBev.




























