
lecturer, Plant Science: Plant Pathology
 Marlese Meiring,
Marlese Meiring,PhD candidate, Plant
Science: Plant Pathology
Part 2: Sclerotinia
The South African Sclerotinia Research Network (SASRN) was established to increase collaboration between the research community and industry on Sclerotinia diseases. Over the past few years, researchers within the SASRN conducted research into aspects important to disease development and management.
This article is part of a series on Sclerotinia research in South Africa, the first of which was published in SA Graan/Grain in September 2021. It focusses on the effects of heat and cattle digestion on the survival of S. sclerotiorum sclerotia.
Ability to survive
Pathogen survival is one of the cornerstones of successful plant disease initiation and development. Sclerotinia sclerotiorum produces sclerotia, melanised masses of fungal hyphae on completion of the disease cycle or when conditions are no longer conducive to disease development. Although these survival structures may have various dimensions, their capacity for survival is significant and can extend up to a decade in and on soil surfaces (Adams & Ayers, 1979; Ben-Yephet et al., 1993).
The McLab Epidemiology group at the University of the Free State (UFS) invested significant research efforts into Sclerotinia stem and head rot, inspired through interactions with producers at SASRN information days. Two questions have been posed by producers relating to the ability of sclerotia to survive. Firstly, would sclerotia germinate if fields were burnt after harvest? Secondly, if they have travelled through the gut of livestock, could they still initiate disease? The research group has been fortunate to be able to elucidate these questions. Marlese Meiring, a PhD candidate under the supervision of Dr Lisa Rothmann, researched these two questions in her research project titled, ‘Sclerotinia sclerotiorum disease potential and management responses in soybean and sunflower’.
Research method
Sclerotia from sunflower cultivar field trials in Delmas were collected and sorted into four size categories: the smallest measured at 15 mm (~0,054 g) in diameter and the largest was greater than 45 mm (~1,310 g). Sclerotia were buried at 5 cm and left on the soil surface, allowing for various cultivation practices to be considered. Thereafter, dry heat temperatures of 125°C, 155°C, 185°C and 200°C were applied for five, ten and fifteen minutes. The viability of sclerotia was determined by placing recovered sclerotia on artificial media and recording the germination responses based on the mycelial growth from the sclerotia.
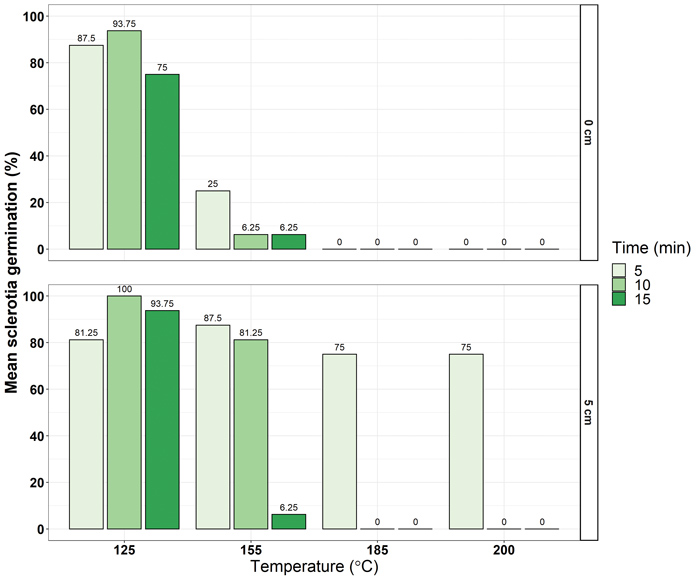
Results
A total of 384 sclerotia across the four weight classes were analysed. Results showed significant differences in germination response among the main effects of sclerotia size category, temperature, duration and burial depth. In addition, significant interactions between the main effects were observed. The smallest sclerotia had a mean germination ability of less than 30%, whereas all sclerotia greater than 0,054 g had a mean germination between ~38% and 42%. Temperatures above 185°C resulted in a mean germination response below 20%, which is promising as field fires are known to exceed this temperature and range between 50°C and 500°C (Mataix-Solera et al., 2011).
As expected, the mean germination response was lower where sclerotia remained on the soil surface (<30%) and when durations of exposure time increased, ranging from ~20% to 30% mean germination from five minute to fifteen minute durations. The most effective treatments were those where sclerotia were exposed to temperatures greater than 185°C for a duration exceeding five minutes, either on the soil surface or buried at 5 cm. Although burning harvested soybean and sunflower fields is not a common practice in South Africa, the burning of crop residues through narrow windrow burning in canola has been practised to manage sclerotial populations in Australia (Hind-Lanoiselet et al., 2005). Additionally, narrow windrow burning minimises the soil to field fire exposure, reducing the detrimental effects of field fire on soil health (Brooks et al., 2018).
21 whole dung samples were collected in 2020 from commercial sunflower fields grazed by cattle in Clocolan in the Free State. Sclerotia were hand-harvested from the dung after it was soaked in water and placed into size categories. Sclerotia germination responses were conducted in the same manner as above, resulting in a measure of pathogen viability. A total of 104 sclerotia were harvested, with average weights from 0,03 g to 0,61 g, although only three sclerotia were collected in the largest weight category. Only two sclerotia, from the 0,03 g and 0,328 g categories, germinated successfully, resulting in a 96,2% successful degradation of sclerotia which passed through the rumen of cattle. These results corroborate similar findings, although reported in 1937, where sclerotia passed through the digestive tract of sheep, resulting in less than 2% germination. Although this provides clarity on sclerotia viability in the digestive system of cattle, producers are urged to remain cognisant when moving livestock between fields infested by sclerotia and those not infected, so as to mitigate the spread of S. sclerotiorum.
The evaluation of whether windrow burning is a viable option for integrated pest management of S. sclerotiorum, requires producers to consider the economic benefits of this practice, particularly where grazing populations of livestock are active.
A word of thanks
We are grateful to the Department of Science and Innovation (DSI), the Oil and Protein Seeds Development Trust (OPDT) and the Sasol Agricultural Trust for the funding to conduct this research – and to Grain SA for the support to initiate the Sclerotinia Network (www.sclerotinia.co.za). Our thanks are extended to producers who actively participate in the SASRN, particularly to Koos Strydom who we work closely with on his farm.
For more information send an email to coetzeela@ufs.ac.za.
References
- Adams, P & Ayers, WA. 1979. Phytopathology, 69: 896 – 899.
- Ben-Yephet, Y et al. 1993. Phytopathology, 83: 509 – 513.
- Brooks, KD et al. 2018. Pest management science, 74: 2594 – 2600.
- Brown, JG. 1937. Phytopathology, 27: 1045 – 1050.
- Hind-Lanoiselet, TL et al. 2005. Australasian Plant Pathology, 34: 311 – 317.
- Mataix-Solera, J et al. 2011. Earth-Science Reviews, 109: 44 – 60.




























